Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 3 by Beatrix Zheng.
Improved hemostasis during acute bleeding and a trend to prevent hematoma were observed after the TGM injection. TGM could be an alternative method to achieve better post-VABB hemostasis.
- large breast tumor
- vacuum assisted breast biopsy
- thrombin-gelatin matrix
1. Introduction
Vacuum-assisted breast biopsy (VABB) for benign breast tumor excision is a developing trend in breast surgery. VABB can retrieve a larger volume of breast tissue than a core needle biopsy. A previous study reported that the false negative rate was only 0.1% among 1512 VABB cases [1]. Thereby, VABB can contribute reliable pathological results.
Although VABB is feasible in clinical practice, complications are inevitable. The most common complication of VABB is hematoma. Other complications, such as postoperative pain, subcutaneous bleeding, skin defects, and pneumothorax, have also been reported [2][3]. The most common procedure for achieving hemostasis in VABB is compression with pressure. Suturing of bleeders are rarely performed during VABB.
Recently, numerous products have been introduced to achieve hemostasis in different ways, such as the thrombin-gelatin matrix (TGM), topical hemostatic agents (HA) (e.g., sponges), thrombin, fibrin glue, and other types of surgical sealants. Several studies have reported the use of TGM compared with other hemostatic agents. TGM has been demonstrated to be an efficacious method to reduce the time to achieve hemostasis and the length of hospital stay, resulting in less consumption of health resources [4][5][6].
The TGM is a hemostatic matrix composed of a bovine gelatin matrix (FLOSEAL, Baxter Healthcare Corporation, Deerfield, IL, USA). TGM has been used in multiple surgical fields, such as cardiac and vascular surgeries, orthopedic surgery, tonsillectomy and adenoidectomy, sinus surgery, thyroidectomy, gynecologic surgery, urologic procedures, and lacrimal surgery [6].
2. Detailed Analysis
Among the 147 patients, a total of 206 breast tumors were removed by 7-gauge ultrasound-guided VABB. The median age of the patients was 38 years (Table 1). The histopathological reports of the 206 lesions revealed that four lesions were malignant (1.9%): two invasive ductal carcinomas and two intraductal carcinomas. The 202 benign lesions were as follows: 114 fibroadenomas (56.4%), 40 fibrocystic lesions (19.8%), 12 benign phyllodes tumors (5.9%), 9 intraductal papilloma (4.4%), 6 usual ductal hyperplasia (3.0%), 6 atypical ductal hyperplasia (3.0%), 7 harmatoma (3.4%), 2 granulomatous mastitis (1.0%), 2 lactating mastitis (1.0%), 2 xanthogranulomatous inflammation (1.0%), and 2 pseudoangiomatous stromal hyperplasias (1.0%). Both groups had two patients with malignancy at the final pathological report. Patients diagnosed with breast malignancy underwent a standard lumpectomy or mastectomy with sentinel lymph node biopsy as definite oncological surgery. No mortality was noted after VABBs.
Table 1. Patient demographics and lesion characteristics.
| Characteristics | All Patients (n = 147) |
|---|
= 0.003). Among patients with hematoma, there was no statistically significant difference between the two groups (25% vs. 26.7%, p = 0.85) (Table 2).
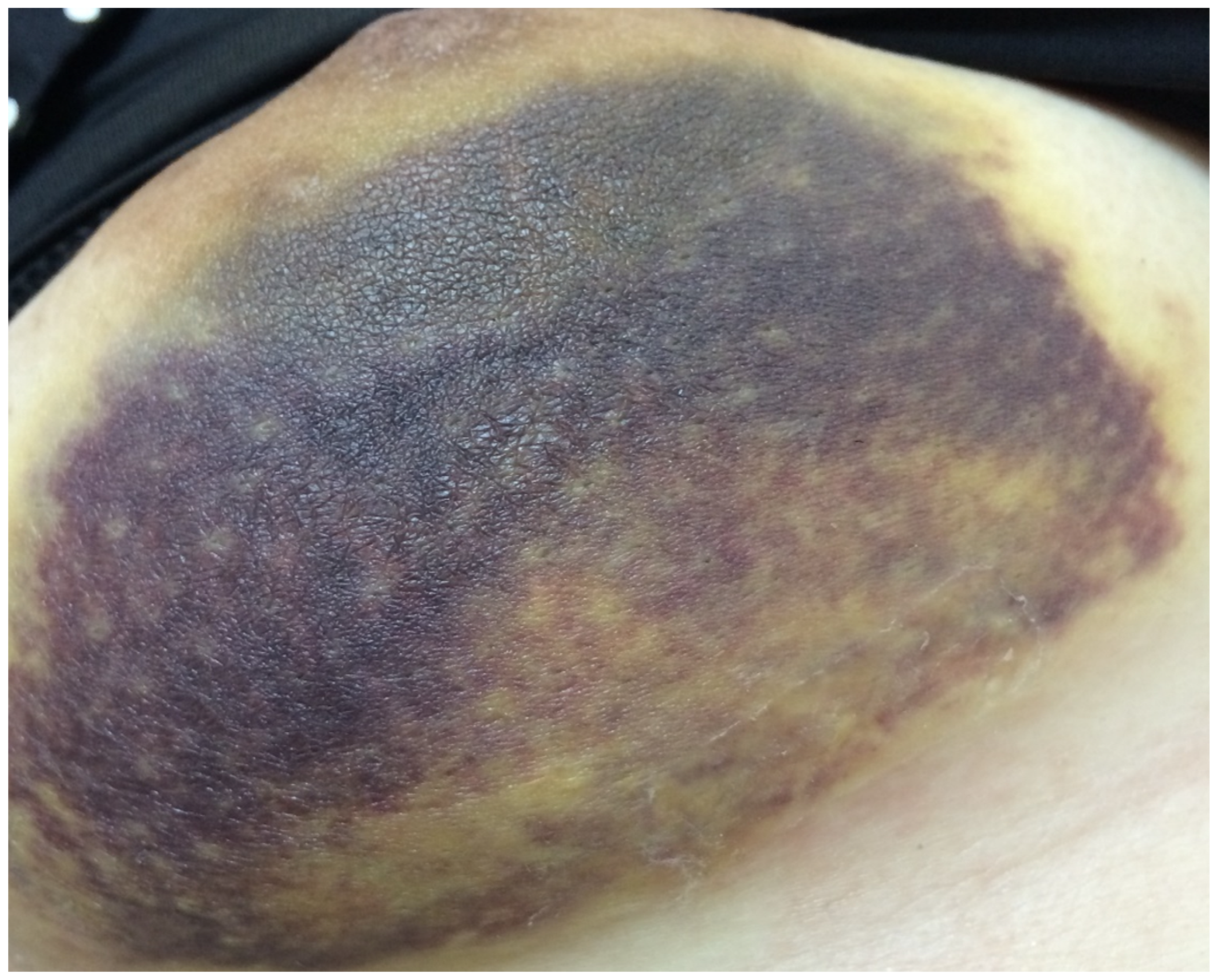

Figure 1. Patient with acute bleeding in Day 1.
Table 2. Hematoma and acute bleeding.
| Complications | Hemostasis with TGM (n = 72) | All Lesion (n = 206) |
Hemostasis with TGM (n = 72) |
Hemostasis without TGM (n = 75) |
p Value |
|---|---|---|---|---|---|
| Hemostasis without TGM | (n = 75) | p Value | |||
| Age |
Table 3. Result of factors affecting Hematoma.
| Parameters | Odds Ratio | 95% CI | p Value | ||
|---|---|---|---|---|---|
| 39 (17–78) | 38 (14–68) | 0.6 | |||
| Size (cm) | |||||
| Lesion number (single/>2) | 1.109 | 0.493–2.497 | 0.802 | ||
| Lesion size (2/> 2 cm) | 2 (2–5) | 0.783 | 0.373–1.644 | 0.5182 (2–4.6) | 0.42 |
| Lesion number | |||||
| Thrombin-gelatin matrix (No/Yes) | 1.108 | 0.528–2.327 | 0.787 |
CI = confidence interval.
Table 4. Result of factors affecting acute bleeding.
| Parameters | Odds Ratio | 95% CI | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Lesion number (single/>2) | 1.557 | 0.514–4.711 | 0.434 | |||||
| Lesion size (2/> 2 cm) | 0.64 | 0.244–1.678 | 0.364 | |||||
| Thrombin-gelatin matrix (No/Yes) | ||||||||
| Post-VABB hematoma | 0.85 | |||||||
| Yes | 18 (25.0%) | 20 (26.7%) | 1.0 | |||||
| No | 54 (75.0%) | 55 (73.3%) | 5.225 | Single | 102 (69.4%) | 50 (69.4%) | 52 (69.3%) | |
| Acute bleeding | 0.003 | Multiple | 45 (30.6%) | 22 (30.6%) | 23 (30.7%) | |||
| Yes | ||||||||
| 4 (5.5%) | Pathology | 1.0 | ||||||
| Benign | 202 (98.1%) | 100 | 102 | |||||
| Malignant | 4 (1.9%) | 2 | 2 |
TGM = Thrombin-gelatin matrix.
A total of 72 patients received hemostasis via TGM and 75 patients received hemostasis by compression. There were four patients (5.5%) with bleeding (Figure 1) and 18 (25%) with hematoma in the TGM group. However, 17 patients (22.7%) had bleeding and 20 (26.7%) had hematoma in the non-TGM group. The rates of postoperative acute bleeding in the TGM group were significantly lower than those in the non-TGM group (5.5% vs. 22.7%, p
| 17 (22.7%) | |
| No | |
| 68 (94.4%) | 58 (77.3%) |
TGM = thrombin-gelatin matrix.
Univariable analyses in hematoma group showed that there were no significant association with lesion number (p = 0.802), lesion size (p = 0.518) or application of TGM (p = 0.787) (Table 3). However, in the acute bleeding group, although there were no significant associations with lesion number (p = 0.434), or lesion size (p = 0.364), the application of TGM was affected independently (p = 0.005) (Table 4). All of hematoma were resolved during the 6-month follow-up.
| 1.648–16.57 |
| 0.005 |
CI= confidence interval.
3. Current Insights
Jung et al. showed that ultrasound-guided core needle biopsy of a benign breast mass measuring 2 cm is sufficient to rule out malignancy, with an accuracy rate of 98.6% [7]. The advantage of VABB is that it provides not only the diagnosis but also the removal of the mass [8]. The most common complication after VABB is hematoma [9]. VABB is a minimally invasive surgery; therefore, neither electrical coagulation nor internal sutures for hemostasis are available. External compression can resolve most of the complications. Fu et al. introduced the effectiveness and safety of using a Foley catheter in VABB to prevent hematoma and bleeding [9][10], which was found to be less time consuming and to result in less bleeding and post-interventional hematoma.
The hematoma and acute bleeding rates were 25.8% (38/147) and 14.2% (21/147), respectively. The result was similar to that reported by Fu et al. [10]. Schaefer et al. reported significantly more hematomas and acute bleedings for the 8-gauge-Mammotome®-system vs. the 11-gauge-Mammotome®-system (35.5% vs. 16.7%, p = 0.029; 41.9% vs. 8.4%, p < 0.001) [11]. Zheng et al. reported a hematoma rate of 11.4% but lacked acute bleeding rate. Zheng et al. also reported that the lesion size and number of lesions were independently associated with hematoma occurrence [12].
Removing larger lesions may lead to increased surgical space and the risk of vessel injury. It is difficult to treat refractory bleeding by external compression of the wound. Therefore, the researchers filled the residual surgical space with TGM injection. Lesion number and size were not associated with hematoma or acute bleeding. However, the result showed that the application of TGM would decrease the rate of acute bleeding independently.
The application of TGM in breast surgery has rarely been reported. Henkel et al. reported the presentation of breast pseudo-microcalcification on mammogram after TGM injection [13]. During these series, postoperative mammograms were performed in 10 patients, and no appearance of pseudo-microcalcification was noted. The researchers suggest yearly to follow-up of the cases due to the possibility of breast pseudo-microcalcification.
In this research, the malignancy rate after VABB was 1.9%. Lee et al. reported a malignancy rate of 5.4% after a 10-year VABB follow-up [1]. In order to expand the indications of VABB, there are several studies presenting VABB for breast cancer excision or biopsy confirmation for post-neoadjuvant chemotherapy [3][14][15]. However, a higher residual tumor rate in early breast cancer patients receiving VABB has been reported [16]. Therefore, it's suggested that VABB is a better indicator of benign breast lesions, such as fibroadenoma, hematoma, lipoma, and benign papilloma. If malignancy is present after VABB, standard oncological surgery is recommended.
References
- Lee, S.H.; Kim, E.K.; Kim, M.J.; Moon, H.J.; Yoon, J.H. Vacuum-assisted breast biopsy under ultrasonographic guidance: Analysis of a 10-year experience. Ultrasonography 2014, 33, 259–266.
- Simon, J.R.; Kalbhen, C.L.; Cooper, R.A.; Flisak, M.E. Accuracy and Complication Rates of US-guided Vacuum-assisted Core Breast Biopsy: Initial Results. Radiology 2000, 215, 694–697.
- Park, H.L.; Hong, J. Vacuum-assisted breast biopsy for breast cancer. Gland Surg. 2014, 3, 120–127.
- Krishnan, S.; Conner, T.M.; Leslie, R.; Stemkowski, S.; Shander, A. Choice of hemostatic agent and hospital length of stay in cardiovascular surgery. Semin. Cardiothorac. Vasc. Anesth. 2009, 13, 225–230.
- Kim, H.J.; Fraser, M.R.; Kahn, B.; Lyman, S.; Figgie, M.P. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: A randomized controlled trial. J. Bone Jt. Surg. Am. 2012, 94, 1160–1165.
- Echave, M.; Oyaguez, I.; Casado, M.A. Use of Floseal®, a human gelatine-thrombin matrix sealant, in surgery: A systematic review. BMC Surg. 2014, 14, 111.
- Jung, H.K.; Moon, H.J.; Kim, M.J.; Kim, E.K. Benign core biopsy of probably benign breast lesions 2 cm or larger: Correlation with excisional biopsy and long-term follow-up. Ultrasonography 2014, 33, 200–205.
- Eller, A.; Janka, R.; Lux, M.; Saake, M.; Schulz-Wendtland, R.; Uder, M.; Wenkel, E. Stereotactic vacuum-assisted breast biopsy (VABB)—A patients’ survey. Anticancer Res. 2014, 34, 3831–3837.
- Lian, Z.Q.; Yu, H.Y.; Zhang, A.Q.; Xie, S.M.; Wang, Q. Use of urinary balloon catheter to prevent postoperative bleeding after ultrasound-guided vacuum-assisted breast biopsy. Breast J. 2020, 26, 144–148.
- Fu, S.M.; Wang, X.M.; Yin, C.Y.; Song, H. Effectiveness of hemostasis with Foley catheter after vacuum-assisted breast biopsy. J. Thorac. Dis. 2015, 7, 1213–1220.
- Schaefer, F.K.; Order, B.M.; Eckmann-Scholz, C.; Strauss, A.; Hilpert, F.; Kroj, K.; Biernath-Wupping, J.; Heller, M.; Jonat, W.; Schaefer, P.J. Interventional bleeding, hematoma and scar-formation after vacuum-biopsy under stereotactic guidance: Mammotome(®)-system 11 g/8 g vs. ATEC(®)-system 12 g/9 g. Eur. J. Radiol. 2012, 81, e739–e745.
- Zheng, J.; Cai, S.; Song, H.; Wang, Y.; Han, X.; Han, G.; Wu, H.; Gao, Z. Prediction of postoperative hematoma occurrence after ultrasound-guided vacuum-assisted breast biopsy in minimally invasive surgery for percutaneous removal of benign breast lesions. Gland Surg. 2020, 9, 1346–1353.
- Henkel, A.; Cooper, R.A.; Ward, K.A.; Bova, D.; Yao, K. Malignant-appearing microcalcifications at the lumpectomy site with the use of FloSeal hemostatic sealant. AJR Am. J. Roentgenol. 2008, 191, 1371–1373.
- Heil, J.; Schaefgen, B.; Sinn, P.; Richter, H.; Harcos, A.; Gomez, C.; Stieber, A.; Hennigs, A.; Rauch, G.; Schuetz, F.; et al. Can a pathological complete response of breast cancer after neoadjuvant chemotherapy be diagnosed by minimal invasive biopsy? Eur. J. Cancer 2016, 69, 142–150.
- Heil, J.; Kummel, S.; Schaefgen, B.; Paepke, S.; Thomssen, C.; Rauch, G.; Ataseven, B.; Grosse, R.; Dreesmann, V.; Kuhn, T.; et al. Diagnosis of pathological complete response to neoadjuvant chemotherapy in breast cancer by minimal invasive biopsy techniques. Br. J. Cancer 2015, 113, 1565–1570.
- He, X.F.; Ye, F.; Wen, J.H.; Li, S.J.; Huang, X.J.; Xiao, X.S.; Xie, X.M. High Residual Tumor Rate for Early Breast Cancer Patients Receiving Vacuum-assisted Breast Biopsy. J. Cancer 2017, 8, 490–496.
More
