Usually, miRNAs function post-transcriptionally, by base-pairing with the 3′UTR of target mRNAs, repressing protein synthesis in the cytoplasm. Furthermore, other regions including gene promoters, as well as coding and 5′UTR regions of mRNAs are able to interact with miRNAs. This entry provides information about miRNAs involvement in the various levels of transcription and translation regulation, as well as discusses therapeutic potential of tumor-suppressor epi-miRs used in in vitro and in vivo anti-cancer therapy.
- epi-miRs
- DNMT inhibitors
- HDAC
- lncRNA
- virial vector
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Although research into RNA biology has been ongoing for more than two decades, almost each year brings new discoveries. Until recently, it was thought that microRNAs (miRNAs) act mainly in the cytoplasm at the post-transcriptional level. Interestingly, miRNAs can exert regulatory effect both in the cell (i.e., cytoplasm and nucleus) in which they are produced and in neighboring cells. The latter intracellular transfer of miRNA is mediated by gap junction channels or exosomes [1]. Interestingly, mature miRNAs can regulate one or more mRNA targets, but also a single mRNA transcript can be bound and regulated by many different miRNAs. It is estimated that each miRNA can recognize ~100–200 target sites of the transcriptome and the inhibitory effect on expression can be achieved at 1000 copies per cell [1][2][1,2]. miRNAs can recognize and bind to 3′UTR, 5′UTR and coding sequence of their targets’ mRNA, as well as to promoter regions. Considering miRNAs variety and localization, cell type and cell state, their possibilities to regulate gene expression are limitless.
2. miRNAs As Potential Cancer Epi-Therapeutics
Over the past few decades growing evidence has linked epigenetic mechanisms with the regulation of gene expression. Epigenetic markers such as DNA methylation and post-translational modifications of histone tails can rearrange the structure of chromatin leading either to activation or repression of transcription activity (for details see reviews [56,57]).
Recently, a subclass of miRNAs, referred to as epi-miRNAs, that influence the expression of genes encoding epigenetic effector and reader proteins, has been identified [3][59]. Due to the important role of epi-miRs in the modulation of the epigenome, they are currently considered as potential therapeutic targets, especially in cancer. Manipulation of epi-miRs can affect the expression of epigenetically-regulated genes, such as oncogenes and/or tumor suppressor genes, involved in important cellular pathways including DNA replication, cell cycle progression and apoptosis [4][5][60,61]. The two types of miRs, oncomiRs and tumor-suppressor miRs, can be distinguished regarding their role in carcinogenesis. Generally, oncomiRs are up-regulated thereby increasing cancer cell proliferation and metastasis, in contrast the expression of tumor-suppressor miRs are down-regulated leading to enhanced tumorigenesis [6][62]. In this review, we focus on the therapeutic potential of tumor-suppressor epi-miRs that are downregulated in various types of cancer (casi el tinc. Emerging studies found that the decreased levels of epi-miRs promote cell proliferation, colony formation, tumor growth and metastasis [6][7][8][62,63,64]. Moreover, the suppression of some epi-miRs are responsible for the drug resistance of cancer cells [8][9][64,65]. Schematic relationship between downregulated tumor-suppressor epi-miRs, chromatin-modifying enzymes and cellular processes is shown in Figure 1Figure 2.
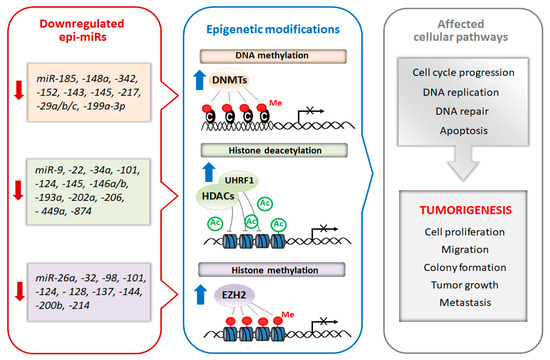
Figure 12.
Epi-miRs-targeted cancer therapy seems to be a promising approach since it is able to influence not only a single gene, but multiple pathways. It is possible to re-establish expression of epi-miRs by delivering synthetic miR mimics (double stranded RNA oligonucleotides directly loaded into RISC) or chemically modified poly(nucleic acids), however, cellular uptake of free synthetic miRs are limited because of the ease in which they a degraded in biofluids [10][76]. In order to overcome poor in vivo stability and improve efficient and specific-site delivery of miRs to the tumor, innovative delivery systems are required. Currently, both viral and non-viral systems are used to increase stability of miRNA oligonucleotides and enhance their therapeutic effect. Administration of epi-miRs via viral vectors (e.g., adenoviruses, adeno-associated viruses (AAV) or lentiviruses) is very effective, as shown by systemic intravenous injection of epi-miR, miR-26a, packaged into AAV vector, which inhibited progression of hepatocellular carcinoma in a mouse model [11][77]. However, due to the viral vectors possible toxicity and immunogenicity their use in clinical practice is limited. In this context, non-viral systems seem to be more promising, because of the control of their molecular composition, ease in manufacturing and relatively low immunogenicity. Different delivery systems including, lipid-based delivery system, synthetic polymers (e.g, polyethyleneimine (PEI)) and naturally occurring polymers (e.g., chitosan, protamine and atelocollagen) are applied to protect miRs from degradation (for details see the review [12][78]). For example, a novel transferrin-conjugated nanoparticle delivery system for synthetic epi-miR, miR-29b, was injected intravenously and significantly prolonged leukemic mice survival [13][79]. Despite significant advances made in delivery systems of miRs, substantial improvements will be necessary for achieving site-specific delivery.
The discovery of therapeutic epi-miRs potential in cancer therapy makes them attractive candidates for next-generation cancer treatment. It therefore seems likely that profiling of miRs expression and then using appropriate epi-miR-based therapeutics may revolutionize cancer treatment by enabling the reversal of the epigenetic program of tumor cells to a more normal state.
3. Conclusions
Knowledge in the miRNA field is steadily increasing and recent information about the mechanisms of action, especially their involvement in epigenetic regulation has shed new light on cellular regulatory networks.
Interestingly, mature miRNAs are present in both the nucleus and the cytoplasm, therefore they can be involved in the regulation of transcription and translation processes. Nuclear miRNAs can influence gene expression via transcriptional activation or transcriptional gene silencing and shaping alternative splicing, Cytoplasmic miRNAs mainly mediated translation inhibition, however, some miRNAs are capable of activating translation of their target mRNA. A growing body of evidence suggests that miRNAs can act as regulators of the cell epigenome through translation inhibition of proteins engaged in epigenetic control and/or interaction with lncRNA. Considering the pervasive role of miRNAs in numerous biological processes, especially tumorigenesis, better understanding of their role in epigenetic regulation will aid the development of new therapeutic strategies.
Currently, miRNA-based treatment approaches for cancer, including tumor-suppressor epi-miRs, are tested in in vitro and in vivo experiments. Although results seem promising further studies will be needed to clarify the safety and effectiveness of epi-miR therapy in clinical practice. We strongly believe that re-introduction of tumor-suppressor epi-miRs will allow for more effective, personalized therapies in the near future.
