You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Rita Xu and Version 2 by Rita Xu.
Drosophila melanogaster has provided a new dimension to the discipline of neuroscience. The research on olfactory and gustatory systems using fruit flies has grown rapidly during the last few years due to the strong genetic similarity that humans share with them.
- Drosophila melanogaster
- gustation
- olfaction
- gustatory receptors
- olfactory receptors
- insect repellents
- ionotropic receptors
1. Introduction
Drosophila melanogaster has provided a new dimension to the discipline of neuroscience. The research on olfactory and gustatory systems using fruit flies has grown rapidly during the last few years due to the strong genetic similarity that humans share with them. This has proved instrumental in establishing D. melanogaster as an ideal model organism. Additionally, fruit flies offer additional advantages to researchers over humans or mammals. The flies can be easily cultured in laboratory conditions and are quite inexpensive to maintain. They produce a large number of eggs and have a relatively short life cycle that provides much-needed flexibility to the researchers. The flies can also be genetically modified [1] and the neurons easily detected in every individual fly [2]. The larval stages of the fly can be used for different experiments, since they are simple in structural organization and easy to handle as compared to adult flies.
Olfaction plays a vital role in the survival of D. melanogaster. The process commences with the binding of volatile odorant molecules to their specific receptors known as odorant receptors (Ors), which are expressed on the olfactory sensory neurons (OSNs). In the larval stages, each of the two dorsal organs (DO) have 21 OSNs [3][4], which contrarily, are contained in specialized sensory hairs known as sensilla, situated on the third antennal segments and maxillary palps in the adult flies. Earlier studies on olfaction in D. melanogaster used odorants in association with an appetitive or an aversive stimulus. The naïve D. melanogaster larvae showed vigorous chemotaxis towards many odorants, including ethyl acetate (EA) [5]. It was observed that the behavioral responses of larvae were diametrically opposite to shorter and longer chain acetates. The former (methyl to pentyl acetates) were attractive in nature, whereas the latter (hexyl to octyl acetates) triggered repulsion [6]. Besides this, the flies effectively avoided odorants which were paired with an electric shock in learning and memory experiments [7]. An age-dependent decline in olfactory response/memory was reported in D. melanogaster [8] and in those offspring born to aging flies [9]. These behavioral assays based on odorants have played a major role in discovering specific genes involved in the olfactory pathways [10][11] and the regions of the insect brain controlling them.
In gustation, there is a remarkable similarity of food choices and their detection that fruit flies share with mammals. This has given opportunities to researchers to conduct in-depth studies of taste perception. Both mammals and fruit flies consume carbohydrates as their major food source, and both avoid chemicals that are either toxic or taste bitter. The sensitivity and range of recognition of gustatory receptors (Grs) of D. melanogaster are similar to mammals. The Grs are mainly localized in the head and pharyngeal regions of the larvae [12], while in adult flies, they are dispersed on the mouthparts, leg tarsi, and around the female ovipositor. The wings of D. melanogaster also possess taste sensilla. The margins of the anterior wings respond to both appetitive and aversive stimuli due to increases in Ca2+ ions in the cytoplasm [13]. The Grs detecting food items are well characterized in D. melanogaster with a single Gr responding to fructose [14], but the case is different in bitter taste receptors. Several researchers have proposed bitter taste Grs to exhibit a multimeric organization comprising of one subunit tuned to a limited specificity, while others function as broadly required co-receptors [15][16][17][18][19][20]. The role of the intestinal gut of fruit flies in perceiving gustatory stimuli has also been reviewed. Due to the presence of Gr transcripts, the intestinal gut controls many biological functions such as ingestion, absorption of nutrients, and balancing body sugar levels [21].
The in-depth understanding of the chemosensory machinery of D. melanogaster has great practical applications, which may be implemented to design new methods of controlling disease-causing insect vectors and crop pests, and to gain a better understanding of how the batches of insect and pest repellents that are currently available in the market function. Insects are one of the major sources of the transmission of pathogens to humans and cattle, as well as the destruction of food crops [22]. Mosquitoes, ticks, sandflies, and mites are a few of the major insect vectors that spread life-threatening diseases to human beings, such as; malaria, dengue, West Nile fever, encephalitis, yellow fever, and Congo hemorrhagic disease [23]. In particular, mosquitoes are the biggest menace to the human population. Anopheles mosquitoes, i.e., An. Gambiae and An. Funestus, are involved in the transmission of the malarial parasite, Plasmodium sp., to their human hosts, triggering one million annual deaths [24][25]. The human filarial nematode Wuchereria bancrofti, and arboviruses spread through the bites of Culex mosquitoes [25] and Aedes aegypti (the yellow fever mosquito), account for many cases of dengue globally [25][26]. As regards crop pests, the facts are disturbing; for example, the spotted wing Drosophila suzukii, an invasive global insect pest, causes huge crop loss by destroying fresh, ripened small fruits and tree fruits at large scale [27][28][29][30].
To present remedies for such problems, the role of chemosensory mechanisms was evaluated in pest management using D. melanogaster as a model organism.
2. Olfactory System—Components and Basic Organization
In D. melanogaster larvae, the olfactory system is mainly localized to the head region. The head portion of larvae has a couple of DOs expressing 21 OSNs in each [3][4] (Figure 1A). Contrarily, the adult fruit flies detect odorants through a pair of antennae and maxillary palps (Figure 1B). These appendages are positioned on the head region, enveloped with numerous sensory hairs called sensilla. The sensilla possess OSNs that are specialized in detecting odorants (Figure 2A). Each antenna has about 410 olfactory sensilla with nearly 1300 OSNs, whereas the number of olfactory sensilla is up to 60 in each maxillary palp with approximately 120 OSNs [31][32][33][34]. These sensory hairs exhibit differences in their morphology. A recent work on serial block-face scanning electron microscopy (SBEM) images of antennal tissues has further led to a systematic morphological and morphometric analysis of the identified olfactory sensilla in D. melanogaster. A plethora of new information, such as inner dendritic enlargement with enhanced mitochondrial content, the presence of extracellular vacuoles in the lumen of sensilla, empty sensilla with no OSNs, and two new unconventional types of basiconic sensory hairs, was discovered. The olfactory sensilla in fruit flies can be separated into four different groups [35] (Figure 2B), which are as follows:
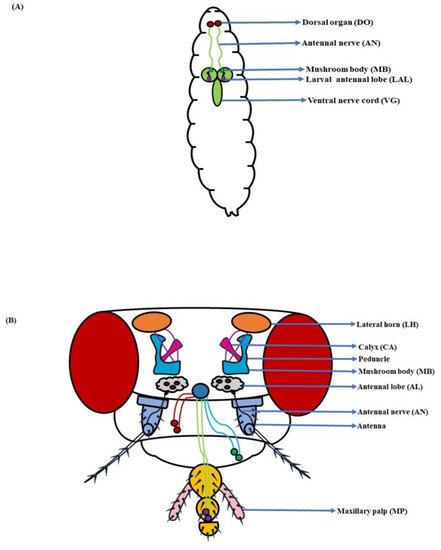
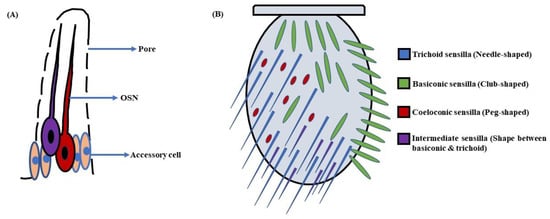

Figure 1. Components of the olfactory system: (A) Larva: With a much smaller and simplified architecture, the dorsal organ (DO), antennal nerve (AN), mushroom body (MB), larval antennal lobe (LAL), and ventral nerve cord (VG) constitute the larval olfactory system; (B) Adult: The antenna and maxillary palp (MP) along with the antennal lobe (AL), antennal nerve (AN), mushroom body (MB), and lateral horn (LH), complete the fly’s olfactory system (Modified from [36][37][38][39]).

Figure 2. Schematic of (A) Sensillum: A sensillum consists of accessory cells, olfactory sensory neurons (OSNs), and pores for odorant molecules to enter; (B) Antenna: The antennae in D. melanogaster’s head are covered with sensory hairs called sensilla. These are of four types, namely trichoid (needle-shaped), basiconic (club-shaped), coeloconic (peg-shaped), and intermediate (shape between basiconic & trichoid) sensilla (Modified from [40]).
a. Basiconic sensilla: The sensory hairs at the proximomedial region of the antenna are club-shaped and are called basiconic sensilla. These sensilla are of 10 types, viz. ab1-ab10, are further grouped into three sub-classes, i.e., large, small, and thin. The large basiconic sensilla (ab1–ab3), ~12 µm in length, house two or four OSNs, whereas small basiconic sensilla (ab7–ab10), which are ~9 µm long, enclose two OSNs. The thin basiconic sensilla (ab4–ab6) are also ~12 µm in length but are thinner in shape. They encompass mostly two with a few cases of four OSNs per sensillum [31][41]. Additionally, there are also two novel types of basiconic sensilla; one is a large basiconic sensillum, abx(3), which encloses three OSNs, and the other is a small basiconic sensillum, abx(1), with a single OSN. Interestingly, one small basiconic sensillum with no OSN is also known to exist, which is designated as abx(0) [35]. The sensory hairs on the maxillary palp are all thin basiconic (pb1–pb3), each housing two OSNs [41]. The basiconic sensilla are single-walled.
b. Trichoid sensilla: The trichoid sensilla are pointed in shape and vary in length from 18–22 µm [31]. They occupy the lateral profile of the antenna, predominantly at the distal tip. These sensilla are of four types, namely at1-at4, and are classified as T1 (at1), T2 (at2), and T3 (at3 & at4) corresponding to the number of OSNs they house, i.e., one, two, and three, respectively [31][41]. However, Fluorescence-guided Single Sensillum Recording (FgSSR) analyses have re-categorized two trichoid sensilla, namely at2 and at3, as intermediate sensilla ai2 and ai3 [42]. Besides this, one trichoid sensillum belonging to the T1 subtype with no OSN has also been found in fruit flies and named T(0) [35]. The trichoid sensilla are also single-walled.
c. Intermediate sensilla: There are a few sensory hairs in the antenna exhibiting length and structure in between trichoid and basiconic sensilla. These are called intermediate sensilla. They are scattered among the trichoids on the frontal antennal surface, with their number varying from 10–20 on each antenna [31][41]. The intermediate sensilla are of two types, namely ai2 and ai3, housing two and three OSNs respectively [42]. They are single-walled.
d. Coeloconic sensilla: The coeloconic sensilla are the smallest of all the sensory hairs. They are short in size (~5 µm) and peg-shaped. Although they are dispersed with other groups of sensilla, the majority of them cover the posterior surface of the antenna. The number of OSNs in coeloconic sensilla varies between two to four neurons per coeloconic sensillum [31][41][43]. The ac3 sensillum houses two OSNs, ac2 and ac4, housing three OSNs each, whereas ac1 compartmentalizes four OSNs [35]. These sensory hairs are double-walled.
Further, in D. melanogaster, the antennal lobe (AL) functions as a deutocerebral neuropil showing functional similarities to the olfactory bulb of the vertebrate brain [44]. At larval stage, the antennal nerve (AN) connects 21 OSNs in DO to the larval antennal lobe (LAL). The LAL has about 30 subunits comparable to the glomerulus of an adult fly [4][45]. The sensory information from LAL then goes through several projections and local neurons (PNs & LNs) to be relayed to higher centers of the larval brain to initiate a behavioral response (Figure 3A). Similarly, in adult flies, OSNs located in the antenna relay sensory information to the AL, consisting of tightly packed neuropils called glomeruli. The number of glomeruli in D. melanogaster is 54 per AL, out of which 52 are innervated by chemosensory and 2 by thermosensory neurons [46][47]. At the glomeruli, the electric signals are further relayed to two specific classes of neurons, the PNs and LNs. The PNs housed in the AL project their axons to the protocerebrum [41] and from here on to the mushroom body (MB) and lateral horn (LH). Thus, the information from the PNs is transferred to these paired neural organs, which comprise higher centers of the fly’s brain [48][49], (Figure 3B).
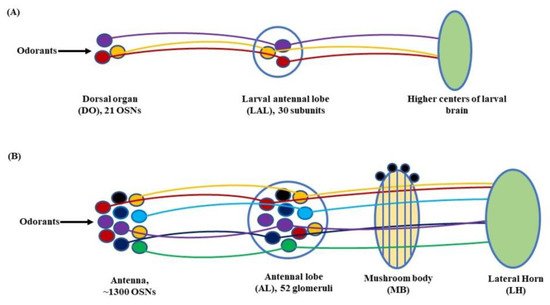

Figure 3. Olfactory signaling pathway of (A) Larva: At the larval stage, the odorant molecules are sensed by the dorsal organ (DO). The stimulus is then transferred to the larval antennal lobe (LAL) by the olfactory sensory neurons (OSNs) housed in the DO. From here onwards, through a grid of projection neurons (PNs) and local neurons (LNs) the electrical signal is projected to the larval brain to elicit a response; (B) Adult: In fruit flies, the olfactory signaling begins with the antennae and maxillary palps. The OSNs, with the help of odorant receptors (Ors), carry the olfactory cue to the antennal lobe (AL). From there on, through a network of PNs and LNs, the stimulus is transferred to the mushroom body (MB) and lateral horn (LH) to initiate a response (Modified from [34][50]).
2.1. Olfactory Sensory Neurons (OSNs)
The OSNs form the most important aspect of olfactory detection in D. melanogaster. Their numbers exhibit great variations between the larval and adult stages of the fly. In larvae there are 21 OSNs per DO, whereas the adult fruit flies house much greater number of neurons. Each antenna displays ~1300 OSNs, whereas the actual number is 120 in each maxillary palp. An individual OSN typically embodies a single Or, although a few multiple receptor OSNs are also present in D. melanogaster [44][51]. The OSNs expressing identical receptor gene/genes then project to a specific glomerulus [52][53], where they synapse onto PNs and LNs [54] for further processing of the olfactory cues. A few studies have suggested that the number of OSNs converging into glomeruli varies between 52–53 OSNs per glomerulus [55]. However, each glomeruli has its own unique neuronal composition and glomerular volume, which is a result of varying numbers of OSNs and uniglomerular PNs received by different sets of glomeruli [56]. Additionally, an increased number of glomeruli per antennal lobe has been reported in DD. melanogaster. melanogaster. The number is now 58, out of which 51 are olfactory [57] and 7, i.e., VP1d, VP1l, VP1m [58], VP2, VP3 [59], and VP4 [60][61][62] are thermo/hygrosensory in nature. They house a total of ~2600 antennal lobe sensory neurons (ALSNs) including OSNs and thermo/hygrosensory neurons (T/HSNs) [63].
2.2. Odorant Receptors (Ors)
The odorant receptors (Ors) in D. melanogaster are membrane-associated proteins by nature. They are encoded and expressed by Or genes [64][65]. The seven transmembrane domains possessed by these proteins are quite distinct and have no homology to the vertebrate GPCRs or Ors [66][67][68]. There are 60 Or genes in D. melanogaster, which encode 62 Ors by the process of alternative splicing (Table 1) [69][70][71]. However, studies using transgenic reporter techniques have changed the scenario to a considerable effect. These studies have revealed that the count of genes exclusive for antennal Ors is 40, whereas 7 genes translate receptors specific to maxillary palps [34][41][52]. The remaining Or genes are involved in the encoding of larval Ors and are not detectably expressed in adult fruit flies. There are 25 larval Ors, of which 13 are specifically expressed in larvae [70][72][73]. Additionally, Ors exhibit a unique feature. The receptors expressed in the antennae and maxillary palps of D. melanogaster are exclusive to them and are not expressed in each other, as established by several algorithmic studies. These studies have identified a dyad element that promotes the expression of maxillary-palp-specific Ors and a motif that represses their expression in the antennae [74]. One more restrictive feature of Ors is that the expression of Or genes in morphologically distinct sensilla follows a set pattern [75]. The study of this pattern has revealed a strong correlation between the developmental pathways of the sensilla and the types of Ors they express [32].
Table 1. Odorant receptors (Ors) in Drosophila melanogaster.
| Olfactory Sensory Neuron | Odorant Receptor | Glomerulus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| - | Or1a * | - | |||||||||
| ai3 | Or2a | DA4m | |||||||||
| Ir21a | Ir7c | Ir56d | Ir20a | Ir11a | Ir20a | Ir7c | Ir7b | Ir7c | ab4A | Or7a | DL5 |
| Ir25a | Ir11a | Ir76b | Ir25a | Ir20a | Ir25a | Ir11a | Ir7c | Ir10a | ab8B | Or9a | VM3 |
| Ir31a | Ir25a | Ir56a | Ir25a | Ir76b | Ir20a | Ir10a | Ir11a | ab1D | Or10a | DL1 | |
| Ir40a | Ir47a | Ir60b | Ir56a | Ir100a | Ir25a | Ir25a | Ir20a | ab6A | Or13a | DC2 | |
| Ir76b | |||||||||||
| Ir41a | Ir56a | Ir60c | Ir76b | Ir47a | Ir52a | Ir25a | ai3A | Or19a | DC1 | ||
| Ir56c | Ir48c | Ir85a | |||||||||
| Ir64a | Ir56b | Ir60d | Ir94a | Ir52a | Ir52b | Ir56a | ai3A | Or19b | DC1 | ||
| Ir60c | Ir51b | ||||||||||
| Ir68a | Ir56d | ||||||||||
| Ir67c | Ir94b | Ir52b | Ir52c | Ir76b | ab3A | Or22a | DM2 | ||||
| Ir76b | Ir60b | ||||||||||
| Ir75a | Ir60c | Ir76b | Ir94c | Ir52c | Ir56a | ab3A | Or22b | DM2 | |||
| Ir94e | Ir60d | ||||||||||
| Ir75b | Ir60d | Ir94a | Ir94h | Ir52d | Ir56d | - | Or22c * | - | |||
| Ir94h | Ir60e | ||||||||||
| Ir75c | Ir76b | Ir94e | Ir56a | Ir60c | ai2B | Or23a | DA3 | ||||
| Ir75d | Ir94b | Ir94f | Ir56d | Ir76b | - | Or24a * | - | ||||
| Ir76a | Ir94e | Ir94h | Ir60c | Ir94e | - | Or30a * | - | ||||
| Ir94b |
. The remaining four Irs, i.e., Ir8a, Ir25a, Ir76b, and Ir93a are present and function as co-receptors in different amalgamations [9391][9492]. This paired functioning of Irs with co-receptors suggests that they work in tandem, resulting in ion channels responding to volatile odorant molecules [9492][9593]. Additionally, the identification of the co-receptor extra loop (CREL), a conserved sequence of the Ir co-receptor ligand-binding domain (LBD) has brought an array of new insights. It has helped in understanding the assembly and stoichiometry of Ir complexes and their intracellular transport in a much-improved way [9694]. Besides olfaction, the Irs are also involved in the perception of gustatory stimuli. A group of approximately 35 Irs, the Ir20a clade is translated in all of the taste organs of the fly co-expressed with bitter or sweet Grs [9795]. This is explained in detail later, in the gustatory section of the review.
Table 3. Ionotropic receptors (Irs) in Drosophila melanogaster (adult).
| Antenna | LTB | LTP | LSO | VCSO | DCSO | Legs | Wings | Abdomen | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ir67b | |||||||||||
| Ir67c | |||||||||||
| Ir76b | |||||||||||
| Ir76b | Ir100a | Ir67a | ab4B | Or33a | DA2 | ||||||
| Ir92a | ab2B, ab5B | Or33b | DM5, DM3 | ||||||||
| Ir94a | pb2A | Or33c | VC1 | ||||||||
| ac3B | Or35a | VC3 | |||||||||
| Ir94g | pb1A | Or42a | VM7d | ||||||||
| Ir100a | ab1A | Or42b | DM1 | ||||||||
| ai3 | Or43a | DA4l | |||||||||
| ab8A | Or43b | VM2 | |||||||||
| - | Or45a * | - | |||||||||
| - | Or45b * | - | |||||||||
| pb2B |
Outline of larval repertoire of ionotropic receptors (Irs). (Adapted from [91]). DO—Dorsal organ, TO—Terminal organ, VO—Ventral organ, DPO—Dorsal pharyngeal organ, DPS—Dorsal pharyngeal sensilla, VPS—Ventral pharyngeal sensilla, PPS—Posterior pharyngeal sensilla.
The Irs confer odorant responses much like the Ors, but more in-depth studies need to be conducted in this direction. The number of Ir genes in the D. melanogaster genome is 61, in addition to 2 putative pseudogenes. Among these, 17 genes encode the antennal Irs, while the rest of them are translated and scattered all over the fly’s body (both in the larval and adult stages); the latter are called divergent Irs. Amid the antenna, out of 13 Irs, the majority of them sense olfactory cues from amines, aldehydes and evaporative acids, and few Irs are hygrosensory or thermosensory in nature [60][8583][9189][9290]
| Ir8a | Ir7a | Ir25a | Ir10a | Ir7a | Ir10a | Ir7a | Ir7a | Ir7a |
| Ir84a | ||||||||
| Or46a | ||||||||
| VA71 | ||||||||
| Ir76b | ||||||||
| ab5B | ||||||||
| Or47a | ||||||||
| DM3 | ||||||||
| at4A | Or47b | VA1v | ||||||
| ab10B | Or49a | DL4 | ||||||
| ab6B | Or49b | VA5 | ||||||
| ab4B | Or56a | DA2 | ||||||
| - | Or59a * | - | ||||||
| ab2A | Or59b | DM4 | ||||||
| pb3A | Or59c | VM7v | ||||||
| - | Or63a * | - | ||||||
| at4B | Or65a | DL3 | ||||||
| at4B | Or65b | DL3 | ||||||
| Ir92a | at4B | Or65c | DL3 | |||||
| ab10A | Or67a | DM6 | ||||||
| ab9 | Or67b | VA3 | ||||||
| ab7B | Or67c | VC4 | ||||||
| at1A | Or67d | DA1 | ||||||
| ab9 | Or69a | D | ||||||
| pb1B | Or71a | VC2 | ||||||
| - | Or74a * | - | ||||||
| ab5A | Or82a | VA6 | ||||||
| - | Or83a * | - | ||||||
| ab, ai, at, pb, ac3 | Or83b/Orco | DA, DC, DL, DM, VA, VC, VM | ||||||
| ai2A | Or83c | DC3 | ||||||
| ab2B | Or85a | DM5 | ||||||
| ab3B | Or85b | VM5d | ||||||
| - | Or85c * | - | ||||||
| pb3B | Or85d | VA4 | ||||||
| pb2A | Or85e | VC1 | ||||||
| ab10B | Or85f | DL4 | ||||||
| at4C | Or88a | VA1d | ||||||
| ab1B | Or92a | VA2 | ||||||
| - | Or94a * | - | ||||||
| - | Or94b * | - | ||||||
| ab7A | Or98a | VM5v | ||||||
| ab6 | Or98b | VM5d |
Outline of odorant receptors (Ors), their OSN classes, and targeted glomeruli. Larval specific Ors are marked with asterisk *. (Adapted from [76][77]).
It is well-established that a solo Or gene is translated in each OSN [41][7876][7977]. However, a few studies have revealed interesting facts about Or gene expression. Each D. melanogaster OSN expressing an Or also expresses a co-receptor named as Orco/Or83b [65][8078]. This co-receptor is essential to the appropriate ciliary routing and functioning of every single Or [65][8179]. The flies devoid of Orco exhibit defective behavioral and electrophysiological responses to a number of odorants [67]. Besides this, four populations of OSNs co-express two conventional Ors (Or33a/Or56a, Or33b/Or47a, Or33b/Or85a and Or33c/Or85e) and a fifth one co-expresses one Or and one Gr (Or10a/Gr10a) along with the co-receptor Orco leading to a modulated ligand response profile [51][52]. A couple of recent works using cryo-electron microscopy have further given an insight into the functioning of Or/Orco heteromeric complexes. Butterwick et al., through structural analyses, has found that an Orco homomer consists of four subunits organized in a symmetrical fashion around a central pore. Each Orco subunit is further composed of seven transmembrane helical segments (S1–S7), out of which S7a (the cytoplasmic section) forms the crux of the anchor domain, whereas S7b (the transmembrane section) contours the central pore. The pore is narrowest at the extracellular end of S7b due to the presence of hydrophobic residues Leu473 and Val469. When a ligand binds to the Or/Orco heteromeric complex, the hydrophobic aperture is expanded. This central ion-conduction pathway then veers into the four lateral channels (6-Å long) that open to the cytosol, providing an uninterrupted pathway for the transfer of ions [8280]. On a similar note, Marmol et al. has deciphered that the odorant receptor MhOr5 in Machilis hrabei exhibits identical quadrivial structural organization and functions similarly to Orco. Here, the binding of the ligand also dilates the S7b helices to gate the ion conduction pathway. In nutshell, these structural understandings of the Or and Orco have shed a light on the promiscuous nature of these receptors, allowing insects to have a versatile chemical recognition system [8381].
2.3. Ionotropic Receptors (Irs)
Besides Ors, an additional group of receptors, called ionotropic receptors (Irs), is also involved in olfaction in D. melanogaster. Structural analyses have revealed their similarities to the ionotropic glutamate receptors (iGluRs) with a homology of less than 34% [8482][8583]. They also share the ion-channel-like characteristic of iGluRs, though the binding site for glutamate is not present [8684][8785]. The chemosensory apparatus of the larval stage is mainly distributed to the head region, encompassing the dorsal organ (DO), terminal organ (TO), ventral organ (VO), dorsal pharyngeal organ (DPO), dorsal pharyngeal sensilla (DPS), ventral pharyngeal sensilla (VPS), and posterior pharyngeal sensilla (PPS), all existing in pairs [45][8886][8987][9088]. All of the above organs express Irs. A few Irs are also present in the body area that forms the complete larval repertoire together with the receptors of the head region (Table 2) [9189]. Contrarily, the distribution of Irs in adult flies is quite diverse (Table 3). In the head region, they are dispersed in the antenna, labral sense organ (LSO), dorsal cibarial sense organ (DCSO), ventral cibarial sense organ (VCSO), and the labellum. The Irs are also expressed in the wings and legs of the fruit fly [9189].
Table 2. Ionotropic receptors (Irs) in Drosophila melanogaster (larva).
| DO | TO | VO | DPO/DPS | VPS | PPS | Abdomen |
|---|---|---|---|---|---|---|
| Ir21a | Ir7a | Ir7g | Ir7a | Ir7b | Ir25a | Ir7d |
| Ir25a | Ir7b | Ir25a | Ir7f | Ir7g | Ir76b | Ir7g |
| Ir68a | Ir7d | Ir67a | Ir7g | Ir25a | Ir92a | Ir10a |
| Ir92a | Ir7e | Ir76b | Ir11a | Ir76b | Ir94g | Ir25a |
| Ir93a | Ir7g | Ir25a | Ir100a | Ir68b | ||
| Ir25a | Ir48b | |||||
| Ir94e | ||||||
| Ir93a | Ir94h |
Outline of distribution of ionotropic receptors (Irs) in adult flies. (Adapted from [91][97]). LTB—Labellar taste bristles, LTP—Labellar taste pegs, LSO—Labral sense organ, VCSO—Ventral cibarial sense organ, DCSO—Dorsal cibarial sense organ.
2.4. Odorant Binding Proteins (Obps)
Encoded by a clan of 52 genes, the Obps are found in abundance in D. melanogaster [9290]. They are a group of highly divergent pint-sized proteins of 13–28 kDa secreted in the olfactory sensillar lymph [9896][9997]. The Obps ease the transportation of hydrophobic odorants such as a few food odorants, pheromones, etc. to the Ors [96][98][10099][101]. The Obp76a, also known as LUSH, is essential for the detection of the pheromone cis-vaccenyl acetate (cVA) by Or67d in trichoid sensilla [102100]. However, various studies have shown that even in the absence of Obp76a, D. melanogaster can detect cVA, refuting its essentiality [101][102]. Another study involving Obp28a, one of the most abundant Obps in fruit flies, showed that it was not involved in the transportation of odorants [103]. Contrary to these findings, recent work has established that Obp28a is essential for the detection of the floral odorant β-ionone by fruit flies [104]. Thus, these findings of the functioning of different Obps suggest that a much larger picture of the exact role and mechanism of their operation remains to be deciphered.
3. Olfactory Signaling Pathway—Interpreting Olfaction in Drosophila melanogaster
3.1. Larva
The larvae and adults of D. melanogaster share a general outline of the olfactory system, though in larvae, it is smaller and simplified. The presence of a reduced number of OSNs in larvae suggests a diminished primary olfactory repertoire as compared to adult flies [105]. The 21 OSNs in the DO are connected to the LAL by the AN. The LAL conother ssists of approximately 30 subunits showing resemblance to the adult glomerulus, though smaller in number and size [4][45]. In the LAL, the OSNs interact with 21 udy involvinniglomerular PNs, 14 multiglomerular PNs, 14 GABAergic and cholinergic LNs, 4 neuromodulatory neurons, 6 subesophageal zone (SEZ) neurons, and 1 descending neuron. The olfactory signals are thus transmitted to higher centers of the larval brain to elicit different behavioral responses [106][107].
3.2. Adult Fly
The antennae and maxillary palps are key to olfaction in adult fruit flies. They house several OSNs that are primarily involved in sensing odorants in D. melanogaster [108]. Studies have revealed that an Or behaves differently to different odorants. It can be excited by some odorants and inhibited by others [109]. Such a response shows the temporally complex nature of odorant signaling. Barring a few cases where the OSNs express either multiple Ors—such as ab5 sensilla, where Or33b and Or47a, are expressed together [51]—or receptors of the gustatory family [110][111], the majority of the 1300 OSNs in the D. melanogaster genome expresses one Or per neuron from the total of 61 Ors [36]. An additional Or, Or83b/Orco functioning as a co-receptor is also expressed in each neuron. It acts in concert with conventional Ors to recognize different odorants [65].
The point of emphasis is this: how do OSNs further process the olfactory stimuli they sense? The answer lies within the antennal lobe. Each of the 52 glomeruli it houses receives projections from the OSNs embodying the same Or [41][46][49][52][60]. From here on, the stimuli are relayed on to a network comprising projection neurons (PNs), inhibitory local neurons (iLNs), and excitatory local neurons (eLNs). The PNs are involved in transmitting olfactory information to higher centers of the fruit fly’s brain, i.e., MBs and LHs, whereas iLNs and eLNs establish lateral connections among different glomeruli [112]. What is interesting at this point is the systemization of the PNs into two different neural tracts. They are the:
a. Inner antennocerebral tract (iACT): the calyx neuropil of the MB, along with the LH, forms synaptic connections with the PNs of the iACT [113].
b. Medial antennocerebral tract (mACT): The PNs of the mACT bypass the MB calyx and go straight to the LH [114]. A few cases have revealed that part of the PNs of the mACT sends its extensions into multiple glomeruli, which trigger an inhibitory response.
This is how olfactory stimuli are perceived by the OSNs and relayed to the higher centers of the brain for further processing, to initiate varying behavioral responses in adult fruit flies.
4. Molecular Basis of Olfactory Signaling—Decoding the Game of Smell
In D. melanogaster, the Or targeting a specific odorant (OrX) forms a heteromeric complex with the universal co-receptor Orco [115]. The mode of action of this complex is synonymous with the functioning of a ligand-activated cation channel [116][117]. On the other hand, the Irs are known to form heteromeric complexes with up to three different subunits. They consist of a variable, odorant-specific Ir (IrX), and one or two broadly expressed co-receptors out of Ir8a, Ir25a, and Ir76b [91][92]. The Irs exhibit a distinct similarity with iGluRs in having a transmembrane done omain, a ligand-binding domain (LBD), and an aperture region [118]. Various models have been extensively studied focusing the moson the functioning of the above-mentioned olfactory receptors in D. melanogaster. These models include the amolecular signaling pathways involved in the sensing of food odorants, pheromones, and CO2, respectively. To have a much better undant erstanding of the cited models, let us divulge the details of these signaling cascades.
4.1. General Odorant Sensing
The odorant-binding proteins (Obps) in frcarry odorant molecules to the OrX-Orco heteromeric complex. The binding of the ligand and receptor leads to the activation of an ion channel [119]. This channel is a non-specific cation channel permeable to Ca2+ and other positively charged ions (Na+, K+). The permeability leads to an influx of catit fliesons changing the membrane potential of the OSNs, leading to their depolarization and repolarization in a subsequent manner [116]. Thus, the olfactory shoignal (electrical activity) is transmitted along the OSNs to the PNs and then to higher centers of D. melanogaster’s brain. This type of signaling is known as ionotropic signaling (Figure 4). In parallel, there are several reports that point toward that is the involvement of G-proteins in odorant detection. It was not infound that the olfactory acumen of flies was altered by mutations targeting the cAMP signaling cascade [120]. The Ors expressed in HEK293 cells, when stimulated by an odorant, resulted in an enhanced cAMP production [121]. A decreased level olf cAMP was also reported to alter the proper detection of odorants by flies [122], whereas odorant stimulation led to an increase in cAMP production [123]. In addition, the role of Gαq [124], Gαo [125], and Gαs in the transmission of odorant stimuli further characterized the involvement of phospholipid in tand cAMP second messengers in olfactory signaling [126]. Still, the involvementrans of G-proteins in odorant detection is still debatable now [127] and is most likely to modulate the functioning of ion channels in one or another way.
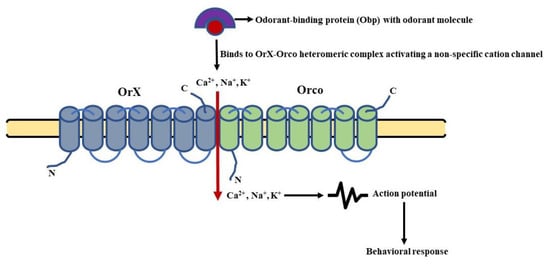
Figure 4. Schematic of ionotropic signaling in general odorant sensing: It involves opening a non-specific cation channel, owing to the binding of odorant molecules to the OrX-Orco heteromeric complex. This leads to a change in the membrane potential and depolarization of the olfactory sensory neurons (OSNs). (Modified from [128]).
4.2. Signaling in Pheromone-Sensing Ors (Prs)
The pherortation of omone molecules attached to the pheromone-binding proteins (PBPs) are fixed to the PrX-Orco heteromeric complex. The SNMP1, i.e., sensory neuron membrane protein 1 most likely associates with the complex and mediates the transfer of pheromones to PrX (pheromone specific Or protein) [129]. The pheromone-receptor complex then activates an ionotropic channel (PrX-Orco channel), leading to an influx of cations (Ca2+, Na+, K+) and subsequent depolarizantstion of the pheromone sensory neurons (PSNs) [105130]. This results in the transmission of the olfactory signal (Figure 5).
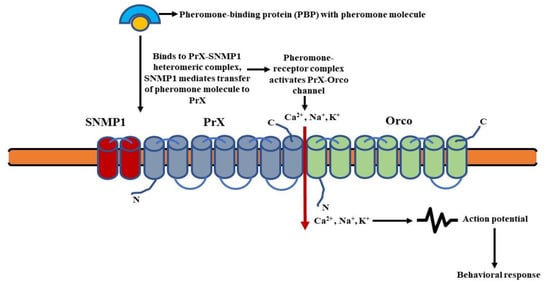
Figure 5. Schematic of ionotropic signaling in pheromone-sensing Ors (Prs): Binding of pheromone molecules to the specific PrX-SNMP1 heteromeric complex results in the activation of an ionotropic channel. This leads to an influx of cations and subsequent depolarization of the pheromone sensory neurons (PSNs). (Modified from [128]).
4.3. Signaling in the Sensing of CO2
The CO2 triggers a strong and inborn evading olfactory behavior in D. melanogaster [131][132]. Its sensing at a concentration of less than ~2% is mediated by the class of OSNs (ab1C) co-expressing Gr21a and Gr63a [131][133][134][135], whereas detection at a higher concentration of more than 5% involves acid-mediating Ir64a and Ir8a [90][136]. A few reports propose that at lower concentrations, the G-proteins might be involved in the detection of CO2 by fruit flies. The Gαq and Gγ30A were found to play a role in the signaling of CO2 in D. melanogaster [127]. Another work hinted that the CO2 receptors Gr21a and Gr63a might activate the TRPC channels through Gαq and PLC21C [137]. However, this is not an established view and more work needs to be conducted in this direction to further validate the involvement of G-proteins in CO2 signaling. At higher levels of CO2, Ir64a and Ir8a act in tandem to decipher a dip in the pH of the sensory lymph to trigger an avoidance behavior [90][136]. Besides this, a different study has put forward the participation of PNs innervating the V-glomerulus (PNvs) in managing the avoidance response of fruit flies to low and high CO2 concentrations. It was shown that the PNv-1pathway is activated at lower concentrations of CO2 (0.5%), whereas higher levels of CO2 (2%) stimulate PNv-1, PNv-2, and GABAergic PNv-3 pathways [138]. Another interesting finding involving the sensing of CO2 by D. melanogaster has come to the light. It has been found that fruit flies, while walking, perceive CO2 as an aversive stimulus, whereas the same stimulus while flying becomes attractive. This in-flight attraction to CO2 involves different components working in tandem, i.e., Ir64a, Orco, and octopamine signaling [139]. However, recent work has Cput forward the fact that fruit flies perceive CO2 as an attractant in both walkintrary to these g and flying conditions when they are in an activated stage of searching for food. This modus operandi is facilitated by the functioning of a distinct chemosensory pathway involving Ir25a [140].
In nutshell, these models highlight the functindingsoning of insect olfactory receptors as heteromeric ligand-gated ion channels. Novel research decoding the structure of Orco has further substantiated the involvement of ion conduction pathways in the perception of olfactory stimuli by insects [80].
5. Olfaction-Based Repellency—The Established and New Players on the Horizon
The process of olfaction is indispensable to the survival of insects. It allows them to detect food, precent obable mates, and the signs of danger so as to avoid them. Likewise, the disease-transmitting insect vectors also use their sense of smell to trace and feed on their human hosts [141][142][143][144]. Additionally, crop infesting insects such as Drosophila suzukii, a major global fruit pest, use olfactory detection of higher CO2 emission to target ripening fruits [145]. This results in a humongous amount of agricultural loss globally, accounting for millions of dollars [145][146][147]. Consequently, targeting and modulating the olfactory system has become a prominent choice for researchers to control the menace of insects and develop better alternatives for combating insect pests.
Insect repellents are still the first line of defense against disease-causing insect vectors. The major ingredient used in these current batches of repellents is N, N-diethyl-m-toluamide (DEET), which triggers an airborne repugnancy among insects [144][148][149]. As a result, DEET is wildly used to ward off blood-sucking has esmosquitoes to limit the transmission of pathogens among humans [148][150]. To understand the molecular machinery blishedehind this olfaction-based repellency caused by DEET, many researchers have opted for D. melanogaster as a model organism. In one of the reseat Obprch works, it was observed that fruit flies with intact antennae avoided DEET-treated food vials. Conversely, flies devoid of both of the antennae, with their maxillary palps unharmed, did not show any repellency to DEET. Thus, it was concluded that the DEET repellency is olfaction driven as antennae house OSNs. To confirm the findings, Or83b/Orco mutant fruit flies were used, as Orco is an indispensable co-receptor required for the proper functioning of Ors [65][67]. The results were consistent, as wild-type flies avoided DEET-treated food vials while Orco mutants were insensitive to DEET. Further in-depth analysis revealed that the reduced acuity of food odorant was related to the electrophysiological inhibition of Or828a/Orco housed in the ab5A sensillum and Or47a/Orco in ab5B sensillum. In summary, it was concluded that DEET reduces the perception of food aroma in fruit flies by inhibiting the odorant-induced activation of a subset of Or/Orco complexes to varying degrees [150]. Similarly, using Orco mutant mosquitoes exhibited that they were not repelled by volatile DEET, indicating the need for an intact Or pathway for DEET repellency [149].
A different work using D. melanogaster also exhibited involvement of the olfactory system in the functioning of DEET. The study revealed that Or42a was involved in the DEET repellency. The receptor was also sensitive to two other major repellents—picaridin and Ir3535—hinting towards is ets generic nature [25]. However, a recent analysisential for the detec with fruit flies has unveiled that, although higher concentrations of DEET were detected by the Orco based olfactory system, there was no specific OSN involved in this process. The deletion of Or42a and Or2a neurons showed no difference in DEET avoidance, pointing towards the involvement of multiple OSNs in the repugnancy of DEET [151]. Altogether, ion of the floral ot can be inferred that the repugnant behavior of insects towards DEET involves their olfactory system. Still, there are several conflicting models involved in DEET olfactory repellency. DEET is found to work by masking odorants. One such example is the reduced attraction of Aedes aegypti mosquitoes to humans by masking the smell of lactic acid [152]. DEET is also known to cause a dip in the rant elease of 1-octen-3-ol, disrupting the host-seeking mechanism of insects by changing the chemical profile of the host’s skin odorants [153][154]. Another hypothesis of the functioning of DEET is that it acts as a confusant. It is likely to alter the regular activation pattern of the glomeruli of insects, thereby blocking the host’s attractiveness. Besides this, as per the smell and avoid model, DEET activates aversive GRNs in insects that override the neural activity of GRNs sensing attractive cues coming from the host. This allow insects to preferably avoid their hosts [155]. In addition to DEET, pyrethrum is also an extremely popular insect repellent that has been used by humans for a long time to control arthropod pests. It is extracted from the dry flowers of the plant Tanacetum cinerariifolium. However, the molecular mode of functioning of this repellent was still a question that needed to be answered. Currently, it has been revealed that there is the involvement of Or7a, Or42b, Or59b, and Or98a in avoidance behavior towards pyrethrum. The first three receptors mentioned above are activated by pyrethrins which are the key insecticidal constituents of pyrethrum, whereas Or98a is stimulated by E-β-farnesene (EBF), a mionone by fnor constituent. It was observed that genetically knocking out Or7a, Or59b, and Or98a obliterated the repellency of fruit flies to pyrethrum, pointing towards their involvement in avoidance behavior [106156].
DEET is a highly efficient insect repellent, with its fair share of problems. First, it needs to be applied on a recurrent basis in increased concentrations making it quite expensive for the people in areas infested with vector-borne diseases [148]. In Thus, these finaddition, a reduced repugnancy by DEET is observed in mosquitoes owing to its repeated exposure. Therefore, there have been efforts from the research community to design and develop better alternatives to DEET, to deal with the menace of insect vectors and pests. A few of the prominent examples are as follows:
a. Geosmin: Trans-1,10-dimethyl-trangs of ts-9-decalol, commonly known as Geosmin is a compound with an earthy smell produced by a few specific groups of bacteria, fungi, and cyanobacteria. Using D. melanogaster as a model organism, it was revealed that at extreme fly low concentrations, Geosmin triggered repellency. Further in-depth analysis exhibited that this aversive behavior was triggered by Or56a housed in the ab4B OSNs [154][157]. Geosmin is also known to induce a dip inctioning o the attraction of fruit flies towards vinegar compounds [157][158]. Thus, the ability of Geosmin to indiffeuce aversion and modulation of inborn attraction makes it a very desirable candidate for a strong repellent.
b. OX1w: In insects, each OSN expressint Og an Or also expresses a co-receptor named Orco [65][78]. This co-receptor is indispensable to the ps roper ciliary routing and functioning of every single Or [65][79]. In D. melanogaster, the attraction towards ethyl acetate (EA) is mediated by the Or42b/Orco heteromeric complex [159]. A group of researchers workingges on fruit fly larvae found that the air-borne application of the Orco antagonist, OX1w, eliminated their chemotactic movement towards EA [144]. Thus, inhibiting that a me functioning of Orco by designing more potent Orco antagonists can be a potential method to control insect vectors and pests.
c. Coffee furanone: 2-methyltetrahydrofuran-3-one, also known as ch largoffee furanone is a natural volatile compound, found to have repellent/attractant properties. A recent work using D. melanogaster showed that the application of coffee furanone triggered an aversive behavior picture in fruit flies. An in-depth analysis brought to light the functions of this compound, as activating the variable OrX subunit of the exact role anOrX/Orco heteromeric complex is necessary for odorant detection. What makes coffee furanone special is its promiscuous nature, as it is known to stimulate nearly 80% of the OSNs in the antennae of D. melanogaster and various other insect mechanism ospecies. In addition, coffee furanone is a common flavoring agent used in food and is considered safe for humans. Consequently, all of these features of coffee furanone brands it a highly desirable candidate to serve as a repellent or attractant to combat insect vectors or crop pests [119].
d. Essential oils: Since it has already been shown that insects use their olfactory their operation remainsystem to detect food and hosts, the use of plant-based essential oils to repel them has immense potential to be developed into high-quality repellents. Several essential oils such as camphor, peppermint oil, norcamphor, nerol, menthyl acetate, menthone, (-)-menthol, etc. have been found to elicit repellency in D. melanogaster and D. suzukii, which are serious to be decipheredglobal crop pests. It was observed that aversion to essential oils was reduced in Orco mutant fruit flies, pointing towards their olfactory mode of functioning. Thus, further in-depth study of the molecular mechanism of the operation of essential oils will be helpful in the long run to ward off the ever-increasing menace of the insects [160] (Figure 6).
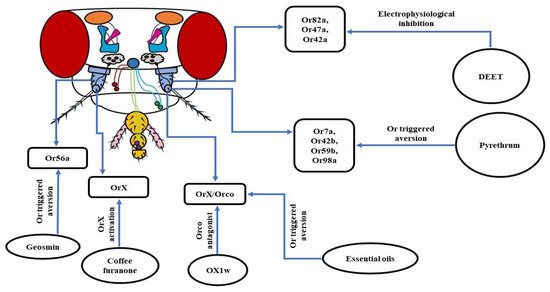
Figure 6. Schematic of molecular players involved in the olfaction-based avoidance of insect repellents: Using D. melanogaster as a model organism it has been observed that the functioning of DEET, pyrethrum, and a slew of new potent alternatives is based on the targeting of either OrX or Orco subunit of the OrX/Orco heteromeric complexes.
References
- Venken, K.J.; Sarrion-Perdigones, A.; Vandeventer, P.J.; Abel, N.S.; Christiansen, A.E.; Hoffman, K.L. Genome engineering: Drosophila melanogaster and beyond. Dev. Biol. 2016, 5, 233–267.
- Zheng, Z.; Lauritzen, J.S.; Perlman, E.; Robinson, C.G.; Nichols, M.; Milkie, D.; Torrens, O.; Price, J.; Fisher, C.B.; Sharifi, N.; et al. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 2018, 174, 730–743.
- Heimbeck, G.; Bugnon, V.; Gendre, N.; Haberlin, C.; Stocker, R.F. Smell and taste perception in Drosophila melanogaster larva: Toxin expression studies in chemosensory neurons. J. Neurosci. 1999, 19, 6599–6609.
- Bose, C.; Basu, S.; Das, N.; Khurana, S. Chemosensory apparatus of Drosophila larvae. Bioinformation 2015, 11, 185–188.
- Monte, P.; Woodard, C.; Ayer, R.; Lilly, M.; Sun, H.; Carlson, J. Characterization of the larval olfactory response in Drosophila and its genetic basis. Behav. Genet. 1989, 19, 267–283.
- Cobb, M.; Danett, F. Multiple genetic control of acetate-induced olfactory responses in Drosophila melanogaster larvae. Heredity 1994, 73, 444–455.
- Yarali, A.; Ehser, S.; Hapil, F.Z.; Huang, J.; Gerber, B. Odour intensity learning in fruit flies. Proc. Biol. Sci. 2009, 276, 3413–3420.
- Tamura, T.; Chiang, A.; Ito, N.; Liu, H.; Horiuchi, J.; Tully, T.; Saitoe, M. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron 2003, 40, 1003–1011.
- Burns, J.G.; Mery, F. Transgenerational memory effect of ageing in Drosophila. J. Evol. Biol. 2010, 23, 678–686.
- Lavagnino, N.; Serra, F.; Arbiza, L.; Dopazo, H.; Hasson, E. Evolutionary genomics of genes involved in olfactory behavior in the Drosophila melanogaster species group. Evol. Bioinform. 2012, 8, 89–104.
- Tunstall, N.E.; Herr, A.; de Bruyne, M.; Warr, C.G. A screen for genes expressed in the olfactory organs of Drosophila melanogaster identifies genes involved in olfactory behaviour. PLoS ONE 2012, 7, e35641.
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral coding of taste. Neuron 2014, 81, 984–1000.
- Raad, H.; Ferveur, J.; Ledger, N.; Capovilla, M.; Robichon, A. Functional gustatory role of chemoreceptors in Drosophila wings. Cell Rep. 2016, 15, 1442–1454.
- Sato, K.; Tanaka, K.; Touhara, K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 11680–11685.
- Lee, Y.; Moon, S.J.; Montell, C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 4495–4500.
- Moon, S.J.; Lee, Y.; Jiao, Y.; Montell, C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 2009, 19, 1623–1627.
- Weiss, L.A.; Dahanukar, A.; Kwon, J.Y.; Banerjee, D.; Carlson, J.R. The molecular and cellular basis of bitter taste in Drosophila. Neuron 2011, 69, 258–272.
- Lee, Y.; Kang, M.J.; Shim, J.; Cheong, C.U.; Moon, S.J.; Montell, C. Gustatory receptors required for avoiding the insecticide L-Canavanine. J. Neurosci. 2012, 32, 1429–1435.
- French, A.; Mitra, A.; Yanagawa, A.; Sellier, M.; Marion-Poll, F. Drosophila bitter taste(s). Front. Integr. Neurosci. 2015, 9, 58.
- Dweck, H.; Carlson, J.R. Molecular logic and evolution of bitter taste in Drosophila. Curr. Biol. 2020, 30, 17–30.e3.
- Park, J.; Kwon, J.Y. Heterogeneous expression of Drosophila gustatory receptors in enteroendocrine cells. PLoS ONE 2011, 6, e29022.
- Paddibhatla, I.; Mishra, R.K. Drosophila as a model for mosquito: Olfactory signals and host seeking behaviour. Curr. Sci. 2016, 110, 44–46.
- Shrestha, B.; Lee, Y. Cellular and molecular mechanisms of DEET toxicity and disease-carrying insect vectors: A review. Genes Genom. 2020, 42, 1131–1144.
- Leal, W.S. The treacherous scent of a human. Nature 2010, 464, 37–38.
- Syed, Z.; Pelletier, J.; Flounders, E.; Chitolina, R.F.; Leal, W.S. Generic insect repellent detector from the fruit fly Drosophila melanogaster. PLoS ONE 2011, 6, e17705.
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496.
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7.
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.; Chu, D.; Daane, K.M.; Gibert, P.; Desneux, N. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494.
- Larson, N.R.; Strickland, J.; Shields, V.D.; Biondi, A.; Zappala, L.; Cavallaro, C.; Colazza, S.; Escudero-Colomar, L.A.; Briem, F.; Vogt, H.; et al. Detection and monitoring of Drosophila suzukii in raspberry and cherry orchards with volatile organic compounds in the USA and Europe. Sci. Rep. 2021, 11, 6860.
- Shaw, B.; Hemer, S.; Cannon, M.; Rogai, F.; Fountain, M.T. Insecticide control of Drosophila suzukii in commercial sweet cherry crops under cladding. Insects 2019, 10, 196.
- Shanbhag, S.R.; Muller, B.; Steinbrecht, R.A. Atlas of olfactory organs of Drosophila melanogaster 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 1999, 28, 377–397.
- Laissue, P.P.; Vosshall, L.B. The Olfactory Sensory Map in Drosophila. Adv. Exp. Med. Biol. 2008, 628, 102–114.
- Martin, F.; Boto, T.; Gomez-Diaz, C.; Alcorta, E. Elements of olfactory reception in adult Drosophila melanogaster. Anat. Rec. 2013, 296, 1477–1488.
- Semaniuk, U. Olfactory system in Drosophila. J. Vasyl Stefanyk Precarpathian Natl. Univ. 2015, 2, 85–92.
- Nava Gonzales, C.; McKaughan, Q.; Bushong, E.A.; Cauwenberghs, K.; Ng, R.; Madany, M.; Ellisman, M.H.; Su, C.Y. Systematic morphological and morphometric analysis of identified olfactory receptor neurons in Drosophila melanogaster. eLife 2021, 10, e69896.
- Keene, A.C.; Waddell, S. Drosophila olfactory memory: Single genes to complex neural circuits. Nat. Rev. Neurosci. 2007, 8, 341–354.
- Kain, P.; Chandrashekaran, S.; Rodrigues, V.; Hasan, G. Drosophila mutants in phospholipid signaling have reduced olfactory responses as adults and larvae. J. Neurogenet. 2009, 23, 303–312.
- Perisse, E.; Burke, C.; Huetteroth, W.; Waddell, S. Shocking revelations and saccharin sweetness in the study of Drosophila olfactory memory. Curr. Biol. 2013, 23, R752–R763.
- El-Keredy, A.; Schleyer, M.; Konig, C.; Ekim, A.; Gerber, B. Behavioural analyses of quinine processing in choice, feeding and learning of larval Drosophila. PLoS ONE 2012, 7, e40525.
- Spletter, M.L.; Luo, L. A new family of odorant receptors in Drosophila. Cell 2009, 136, 23–25.
- Couto, A.; Alenius, M.; Dickson, B.J. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 2005, 15, 1535–1547.
- Lin, C.C.; Potter, C.J. Re-Classification of Drosophila melanogaster trichoid and intermediate sensilla using fluorescence-guided single sensillum recording. PLoS ONE 2015, 10, e0139675.
- Vulpe, A.; Kim, H.S.; Ballou, S.; Wu, S.T.; Grabe, V.; Nava Gonzales, C.; Liang, T.; Sachse, S.; Jeanne, J.M.; Su, C.Y.; et al. An ammonium transporter is a non-canonical olfactory receptor for ammonia. Curr. Biol. 2021, 31, 3382–3390.e7.
- Wilson, R.I. Early olfactory processing in Drosophila: Mechanisms and principles. Annu. Rev. Neurosci. 2013, 36, 217–241.
- Python, F.; Stocker, R.F. Adult-like complexity of the larval antennal lobe of D. melanogaster despite markedly low numbers of odorant receptor neurons. J. Comp. Neurol. 2002, 445, 374–387.
- Grabe, V.; Strutz, A.; Baschwitz, A.; Hansson, B.S.; Sachse, S. Digital in vivo 3D atlas of the antennal lobe of Drosophila melanogaster. J. Comp. Neurol. 2015, 523, 530–544.
- Gallio, M.; Ofstad, T.A.; Macpherson, L.J.; Wang, J.W.; Zuker, C.S. The coding of temperature in the Drosophila brain. Cell 2011, 144, 614–624.
- Masse, N.Y.; Turner, G.C.; Jefferis, G.S. Olfactory information processing in Drosophila. Curr. Biol. 2009, 19, R700–R713.
- Seki, Y.; Dweck, H.; Rybak, J.; Wicher, D.; Sachse, S.; Hansson, B.S. Olfactory coding from the periphery to higher brain centers in the Drosophila brain. BMC Biol. 2017, 15, 56.
- Ramaekers, A.; Magnenat, E.; Marin, E.C.; Gendre, N.; Jefferis, G.S.; Luo, L.; Stocker, R.F. Glomerular maps without cellular redundancy at successive levels of the Drosophila larval olfactory circuit. Curr. Biol. 2005, 15, 982–992.
- Goldman, A.L.; van der Goes van Naters, W.; Lessing, D.; Warr, C.G.; Carlson, J.R. Coexpression of two functional odor receptors in one neuron. Neuron 2005, 45, 661–666.
- Fishilevich, E.; Vosshall, L.B. Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 2005, 15, 1548–1553.
- Semmelhack, J.L.; Wang, J.W. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature 2009, 59, 218–223.
- Rybak, J.; Talarico, G.; Ruiz, S.; Arnold, C.; Cantera, R.; Hansson, B.S. Synaptic circuitry of identified neurons in the antennal lobe of Drosophila melanogaster. J. Comp. Neurol. 2016, 524, 1920–1956.
- Tobin, W.F.; Wilson, R.I.; Lee, W.A. Wiring variations that enable and constrain neural computation in a sensory microcircuit. eLife 2017, 6, e24838.
- Grabe, V.; Baschwitz, A.; Dweck, H.; Lavista-Llanos, S.; Hansson, B.S.; Sachse, S. Elucidating the neuronal architecture of olfactory glomeruli in the Drosophila antennal lobe. Cell Rep. 2016, 16, 3401–3413.
- Bates, A.S.; Manton, J.D.; Jagannathan, S.R.; Costa, M.; Schlegel, P.; Rohlfing, T.; Jefferis, G.S. The Natverse, a versatile toolbox for combining and analysing neuroanatomical data. eLife 2020, 9, e53350.
- Marin, E.C.; Buld, L.; Theiss, M.; Sarkissian, T.; Roberts, R.; Turnbull, R.; Tamimi, I.; Pleijzier, M.W.; Laursen, W.J.; Drummond, N.; et al. Connectomics analysis reveals first-, second-, and third-order thermosensory and hygrosensory neurons in the adult Drosophila brain. Curr. Biol. 2020, 30, 3167–3182.e4.
- Stocker, R.F.; Lienhard, M.C.; Borst, A.; Fischbach, K.F. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990, 262, 9–34.
- Silbering, A.F.; Rytz, R.; Grosjean, Y.; Abuin, L.; Ramdya, P.; Jefferis, G.S.; Benton, R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 2011, 31, 13357–13375.
- Frank, D.D.; Enjin, A.; Jouandet, G.C.; Zaharieva, E.E.; Para, A.; Stensmyr, M.C.; Gallio, M. Early integration of temperature and humidity stimuli in the Drosophila brain. Curr. Biol. 2017, 27, 2381–2388.e4.
- Knecht, Z.A.; Silbering, A.F.; Cruz, J.; Yang, L.; Croset, V.; Benton, R.; Garrity, P.A. Ionotropic receptor-dependent moist and dry cells control hygrosensation in Drosophila. eLife 2017, 6, e26654.
- Schlegel, P.; Bates, A.S.; Sturner, T.; Jagannathan, S.R.; Drummond, N.; Hsu, J.; Serratosa Capdevila, L.; Javier, A.; Marin, E.C.; Barth-Maron, A.; et al. Information flow, cell types and stereotypy in a full olfactory connectome. eLife 2021, 10, e66018.
- Dobritsa, A.A.; van der Goes van Naters, W.; Warr, C.G.; Steinbrecht, A.; Carlson, J.R. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 2003, 37, 827–841.
- Larsson, M.C.; Domingos, A.I.; Jones, W.D.; Chiappe, M.E.; Amrein, H.; Vosshall, L.B. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 2004, 43, 703–714.
- Clyne, P.J.; Warr, C.G.; Freeman, M.R.; Lessing, D.; Kim, J.; Carlson, J.R. A novel family of divergent seven-transmembrane proteins: Candidate odorant receptors in Drosophila. Neuron 1999, 22, 327–338.
- Benton, R.; Sachse, S.; Michnik, S.W.; Voshall, L.B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006, 4, e20.
- Saberi, M.; Seyed-allaei, H. Odorant receptors of Drosophila are sensitive to the molecular volume of odorants. Sci. Rep. 2016, 6, 25103.
- Robertson, H.M.; Warr, C.G.; Carlson, J.R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2003, 100, 14537–14542.
- Joseph, R.M.; Carlson, J.R. Drosophila chemoreceptors: A molecular interface between the chemical world and the brain. Trends Genet. 2015, 31, 683–695.
- Hickner, P.V.; Rivaldi, C.L.; Johnson, C.M.; Siddappaji, M.; Raster, G.J.; Syed, Z. The making of a pest: Insights from the evolution of chemosensory receptor families in a pestiferous and invasive fly, Drosophila suzukii. BMC Genom. 2016, 17, 648.
- Fishilevich, E.; Domingos, A.; Asahina, K.; Naef, F.; Vosshall, L.B.; Louis, M. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr. Biol. 2005, 15, 2086–2096.
- Kreher, S.A.; Kwon, J.Y.; Carlson, J.R. The molecular basis of odor coding in the Drosophila larva. Neuron 2005, 46, 445–456.
- Ray, A.; van Naters, W.V.; Shiraiwa, T.; Carlson, J.R. Mechanisms of odor receptor gene choice in Drosophila. Neuron 2007, 53, 353–369.
- Fuss, S.H.; Ray, A. Mechanisms of odorant receptor gene choice in Drosophila and vertebrates. Mol. Cell. Neurosci. 2009, 41, 101–112.
- Guo, S.; Kim, J. Molecular evolution of Drosophila odorant receptor genes. Mol. Biol. Evol. 2007, 24, 1198–1207. Vosshall, L.B.; Wong, A.M.; Axel, R. An olfactory sensory map in the fly brain. Cell 2000, 102, 147–159.
- Munch, D.; Galizia, C.G. DoOR 2.0—Comprehensive mapping of Drosophila melanogaster odorant responses. Sci. Rep. 2016, 6, 21841. Gonzalez, A.; Jafari, S.; Zenere, A.; Alenius, M.; Altafini, C. Thermodynamic model of gene regulation for the Or59b olfactory receptor in Drosophila. PLoS Comput. Biol. 2019, 15, e1006709.
- Vosshall, L.B.; Wong, A.M.; Axel, R. An olfactory sensory map in the fly brain. Cell 2000, 102, 147–159. Bahk, S.; Jones, W.D. Insect odorant receptor trafficking requires calmodulin. BMC Biol. 2016, 14, 83.
- Gonzalez, A.; Jafari, S.; Zenere, A.; Alenius, M.; Altafini, C. Thermodynamic model of gene regulation for the Or59b olfactory receptor in Drosophila. PLoS Comput. Biol. 2019, 15, e1006709. Stengl, M.; Funk, N.W. The role of the coreceptor Orco in insect olfactory transduction. J. Comp. Physiol. A 2013, 199, 897–909.
- Bahk, S.; Jones, W.D. Insect odorant receptor trafficking requires calmodulin. BMC Biol. 2016, 14, 83. Butterwick, J.A.; del Marmol, J.; Kim, K.H.; Kahlson, M.A.; Rogow, J.A.; Walz, T.; Ruta, V. Cryo-EM structure of the insect olfactory receptor Orco. Nature 2018, 560, 447–452.
- Stengl, M.; Funk, N.W. The role of the coreceptor Orco in insect olfactory transduction. J. Comp. Physiol. A 2013, 199, 897–909. Del Marmol, J.; Yedlin, M.A.; Ruta, V. The structural basis of odorant recognition in insect olfactory receptors. Nature 2021, 597, 126–131.
- Butterwick, J.A.; del Marmol, J.; Kim, K.H.; Kahlson, M.A.; Rogow, J.A.; Walz, T.; Ruta, V. Cryo-EM structure of the insect olfactory receptor Orco. Nature 2018, 560, 447–452. Croset, V.; Rytz, R.; Cummins, S.F.; Budd, A.; Brawand, D.; Kaessmann, H.; Gibson, T.J.; Benton, R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010, 6, e1001064.
- Del Marmol, J.; Yedlin, M.A.; Ruta, V. The structural basis of odorant recognition in insect olfactory receptors. Nature 2021, 597, 126–131. Rytz, R.; Croset, V.; Benton, R. Ionotropic Receptors (IRs): Chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 2013, 43, 888–897.
- Croset, V.; Rytz, R.; Cummins, S.F.; Budd, A.; Brawand, D.; Kaessmann, H.; Gibson, T.J.; Benton, R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010, 6, e1001064. Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162.
- Rytz, R.; Croset, V.; Benton, R. Ionotropic Receptors (IRs): Chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 2013, 43, 888–897. Rimal, S.; Lee, Y. The multidimensional ionotropic receptors of Drosophila melanogaster. Insect Mol. Biol. 2018, 27, 1–7.
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. Singh, R.N.; Singh, K. Fine structure of the sensory organs of Drosophila melanogaster Meigen larva (Dipter: Drosophilidae). Int. J. Insect Morphol. Embryol. 1984, 13, 255–273.
- Rimal, S.; Lee, Y. The multidimensional ionotropic receptors of Drosophila melanogaster. Insect Mol. Biol. 2018, 27, 1–7. Gendre, N.; Luer, K.; Friche, S.; Grillenzoni, N.; Ramaekers, A.; Technau, G.M.; Stocker, R.F. Integration of complex larval chemosensory organs into the adult nervous system of Drosophila. Development 2004, 131, 83–92.
- Singh, R.N.; Singh, K. Fine structure of the sensory organs of Drosophila melanogaster Meigen larva (Dipter: Drosophilidae). Int. J. Insect Morphol. Embryol. 1984, 13, 255–273. Stewart, S.; Koh, T.; Ghosh, A.C.; Carlson, J.R. Candidate ionotropic taste receptors in the Drosophila larva. Proc. Natl. Acad. Sci. USA 2015, 112, 4195–4201.
- Gendre, N.; Luer, K.; Friche, S.; Grillenzoni, N.; Ramaekers, A.; Technau, G.M.; Stocker, R.F. Integration of complex larval chemosensory organs into the adult nervous system of Drosophila. Development 2004, 131, 83–92. Sanchez-Alcaniz, J.A.; Silbering, A.F.; Croset, V.; Zappia, G.; Sivasubramaniam, A.K.; Abuin, L.; Sahai, S.Y.; Munch, D.; Steck, K.; Auer, T.O.; et al. An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing. Nat. Commun. 2018, 9, 4252.
- Stewart, S.; Koh, T.; Ghosh, A.C.; Carlson, J.R. Candidate ionotropic taste receptors in the Drosophila larva. Proc. Natl. Acad. Sci. USA 2015, 112, 4195–4201. Ai, M.; Min, S.; Grosjean, Y.; Leblanc, C.; Bell, R.; Benton, R.; Suh, G.S.B. Acid sensing by the Drosophila olfactory system. Nature 2010, 468, 691–695.
- Sanchez-Alcaniz, J.A.; Silbering, A.F.; Croset, V.; Zappia, G.; Sivasubramaniam, A.K.; Abuin, L.; Sahai, S.Y.; Munch, D.; Steck, K.; Auer, T.O.; et al. An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing. Nat. Commun. 2018, 9, 4252. Vulpe, A.; Menuz, K. Ir76b is a co-receptor for amine responses in Drosophila olfactory neurons. Front. Cell. Neurosci. 2021, 15, 759238.
- Ai, M.; Min, S.; Grosjean, Y.; Leblanc, C.; Bell, R.; Benton, R.; Suh, G.S.B. Acid sensing by the Drosophila olfactory system. Nature 2010, 468, 691–695. Abuin, L.; Bargeton, B.; Ulbrich, M.H.; Isacoff, E.Y.; Kellenberger, S.; Benton, R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 2011, 69, 44–60.
- Vulpe, A.; Menuz, K. Ir76b is a co-receptor for amine responses in Drosophila olfactory neurons. Front. Cell. Neurosci. 2021, 15, 759238. Ai, M.; Blais, S.; Park, J.; Min, S.; Neubert, T.A.; Suh, G.S.B. Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J. Neurosci. 2013, 33, 10741–10749.
- Abuin, L.; Bargeton, B.; Ulbrich, M.H.; Isacoff, E.Y.; Kellenberger, S.; Benton, R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 2011, 69, 44–60. Abuin, L.; Prieto-Godino, L.L.; Pan, H.; Gutierrez, C.; Huang, L.; Jin, R.; Benton, R. In vivo assembly and trafficking of olfactory ionotropic receptors. BMC Biol. 2019, 17, 34.
- Ai, M.; Blais, S.; Park, J.; Min, S.; Neubert, T.A.; Suh, G.S.B. Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J. Neurosci. 2013, 33, 10741–10749. Koh, T.W.; He, Z.; Gorur-Shandilya, S.; Menuz, K.; Larter, N.K.; Stewart, S.; Carlson, J.R. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron 2014, 83, 850–865.
- Abuin, L.; Prieto-Godino, L.L.; Pan, H.; Gutierrez, C.; Huang, L.; Jin, R.; Benton, R. In vivo assembly and trafficking of olfactory ionotropic receptors. BMC Biol. 2019, 17, 34. Menuz, K.; Larter, N.K.; Park, J.; Carlson, J.R. An RNA-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. PLoS Genet. 2014, 10, e1004810.
- Koh, T.W.; He, Z.; Gorur-Shandilya, S.; Menuz, K.; Larter, N.K.; Stewart, S.; Carlson, J.R. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron 2014, 83, 850–865. Shanbhag, S.R.; Hekmat-Scafe, D.; Kim, M.S.; Park, S.K.; Carlson, J.R.; Pikielny, C.; Smith, D.P.; Steinbrecht, R.A. Expression mosaic of odorant-binding proteins in Drosophila olfactory organs. Microsc. Res. Tech. 2001, 55, 297–306.
- Menuz, K.; Larter, N.K.; Park, J.; Carlson, J.R. An RNA-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. PLoS Genet. 2014, 10, e1004810. Graham, L.A.; Davies, P.L. The odorant-binding proteins of Drosophila melanogaster: Annotation and characterization of a divergent gene family. Gene 2002, 292, 43–55.
- Shanbhag, S.R.; Hekmat-Scafe, D.; Kim, M.S.; Park, S.K.; Carlson, J.R.; Pikielny, C.; Smith, D.P.; Steinbrecht, R.A. Expression mosaic of odorant-binding proteins in Drosophila olfactory organs. Microsc. Res. Tech. 2001, 55, 297–306. Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391.
- Graham, L.A.; Davies, P.L. The odorant-binding proteins of Drosophila melanogaster: Annotation and characterization of a divergent gene family. Gene 2002, 292, 43–55. Xu, P.; Atkinson, R.; Jones, D.N.M.; Smith, D.P. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 2005, 45, 193–200.
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. Benton, R.; Vannice, K.S.; Vosshall, L.B. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 2007, 450, 289–293.
- Xu, P.; Atkinson, R.; Jones, D.N.M.; Smith, D.P. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 2005, 45, 193–200. Gomez-Diaz, C.; Reina, J.H.; Cambillau, C.; Benton, R. Ligands for pheromone-sensing neurons are not conformationally activated odorant binding proteins. PLoS Biol. 2013, 11, e1001546.
- Benton, R.; Vannice, K.S.; Vosshall, L.B. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 2007, 450, 289–293. Larter, N.K.; Sun, J.S.; Carlson, J.R. Organization and function of Drosophila odorant binding proteins. eLife 2016, 5, e20242.
- Gomez-Diaz, C.; Reina, J.H.; Cambillau, C.; Benton, R. Ligands for pheromone-sensing neurons are not conformationally activated odorant binding proteins. PLoS Biol. 2013, 11, e1001546. Gonzalez, D.; Rihani, K.; Neiers, F.; Poirier, N.; Fraichard, S.; Gotthard, G.; Chertemps, T.; Maibeche, M.; Ferveur, J.F.; Briand, L. The Drosophila odorant-binding protein 28a is involved in the detection of the floral odour ß-ionone. Cell. Mol. Life Sci. 2020, 77, 2565–2577.
- Larter, N.K.; Sun, J.S.; Carlson, J.R. Organization and function of Drosophila odorant binding proteins. eLife 2016, 5, e20242. Stocker, R.F. The olfactory pathway of adult and larval Drosophila-Conservation or adaptation to stage-specific needs? Ann. N. Y. Acad. Sci. 2009, 1170, 482–486.
- Gonzalez, D.; Rihani, K.; Neiers, F.; Poirier, N.; Fraichard, S.; Gotthard, G.; Chertemps, T.; Maibeche, M.; Ferveur, J.F.; Briand, L. The Drosophila odorant-binding protein 28a is involved in the detection of the floral odour ß-ionone. Cell. Mol. Life Sci. 2020, 77, 2565–2577. Berck, M.E.; Khandelwal, A.; Claus, L.; Hernandez-Nunez, L.; Si, G.; Tabone, C.J.; Li, F.; Truman, J.W.; Fetter, R.D.; Louis, M.; et al. The wiring diagram of a glomerular olfactory system. eLife 2016, 5, e14859.
- Clark, D.A.; Odell, S.R.; Armstrong, J.M.; Turcotte, M.; Kohler, D.; Mathis, A.; Schmidt, D.R.; Mathew, D. Behavior responses to chemical and optogenetic stimuli in Drosophila larvae. Front. Behav. Neurosci. 2018, 12, 324.
- Dweck, H.K.; Ebrahim, S.A.; Khallaf, M.A.; Koenig, C.; Farhan, A.; Stieber, R.; Weibflog, J.; Svatos, A.; Grosse-Wilde, E.; Knaden, M.; et al. Olfactory channels associated with the Drosophila maxillary palp mediate short- and long-range attraction. eLife 2016, 5, e14925.
- Su, C.; Menuz, K.; Carlson, J.R. Olfactory Perception: Receptors, cells, and circuits. Cell 2009, 139, 45–59.
- Scott, K.; Brady, R.; Cravchik, A.; Morozov, P.; Rzhetsky, A.; Zuker, C.; Axel, R. A chemosensory gene family encoding candidate gustatory and olfactory receptor in Drosophila. Cell 2001, 104, 661–673.
- Getahun, M.N.; Wicher, D.; Hansson, B.S.; Olsson, S.B. Temporal response dynamics of Drosophila olfactory sensory neurons depends on receptor type and response polarity. Front. Cell. Neurosci. 2012, 6, 54.
- Huang, J.; Zhang, W.; Qiao, W.; Hu, A.; Wang, Z. Functional connectivity and selective odor responses of excitatory local interneurons in Drosophila antennal lobe. Neuron 2010, 67, 1021–1033.
- Tanaka, N.K.; Awasaki, T.; Shimada, T.; Ito, K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr. Biol. 2004, 14, 449–457.
- Marin, E.C.; Jefferis, G.X.S.E.; Komiyama, T.; Zhu, H.; Luo, L. Representation of the glomerular olfactory map in the Drosophila brain. Cell 2002, 109, 243–255.
- Mukunda, L.; Lavista-Llanos, S.; Hansson, B.S.; Wicher, D. Dimerisation of the Drosophila odorant coreceptor Orco. Front. Cell. Neurosci. 2014, 8, 261.
- Sato, K.; Pellegrino, M.; Nakagawa, T.; Nakagawa, T.; Vosshall, L.B.; Touhara, K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 2008, 452, 1002–1006.
- Zufall, F.; Domingos, A.I. The structure of Orco and its impact on our understanding of olfaction. J. Gen. Physiol. 2018, 150, 1602–1605.
- van Giesen, L.; Garrity, P.A. More than meets the IR: The expanding roles of variant ionotropic glutamate receptors in sensing odor, taste, temperature and moisture. F1000research 2017, 6, 1753.
- Batra, S.; Corcoran, J.; Zhang, D.D.; Pal, P.; Umesh, K.P.; Kulkarni, R.; Lofstedt, C.; Sowdhamini, R.; Olsson, S.B. A functional agonist of insect olfactory receptors: Behavior, physiology and structure. Front. Cell. Neurosci. 2019, 13, 134.
- Martin, F.; Charro, M.J.; Alcorta, E. Mutations affecting the cAMP transduction pathway modify olfaction in Drosophila. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 2001, 187, 359–370.
- Wicher, D.; Schafer, R.; Bauernfeind, R.; Stensmyr, M.C.; Heller, R.; Heinemann, S.H.; Hansson, B.S. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 2008, 452, 1007–1011.
- Murmu, M.S.; Martin, J.R. Interaction between cAMP and intracellular Ca(2+)-signaling pathways during odor-perception and adaptation in Drosophila. Biochim. Biophys. Acta 2016, 1863, 2156–2174.
- Miazzi, F.; Hansson, B.S.; Wicher, D. Odor-induced cAMP production in Drosophila melanogaster olfactory sensory neurons. J. Exp. Biol. 2016, 219 Pt 12, 1798–1803.
- Kain, P.; Chakraborty, T.S.; Sundaram, S.; Siddiqi, O.; Rodrigues, V.; Hasan, G. Reduced odor responses from antennal neurons of G(q)alpha, phospholipase Cbeta, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 4745–4755.
- Chatterjee, A.; Roman, G.; Hardin, P.E. Go contributes to olfactory reception in Drosophila melanogaster. BMC Physiol. 2009, 9, 22.
- Deng, Y.; Zhang, W.; Farhat, K.; Oberland, S.; Gisselmann, G.; Neuhaus, E.M. The stimulatory Gα protein is involved in olfactory signal transduction in Drosophila. PLoS ONE 2011, 6, e18605.
- Yao, C.A.; Carlson, J.R. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J. Neurosci. 2010, 30, 4562–4572.
- Wicher, D. Olfactory signaling in insects. Prog. Mol. Biol. Transl. Sci. 2014, 130, 37–54.
- Gomez-Diaz, C.; Bargeton, B.; Abuin, L.; Bukar, N.; Reina, J.H.; Bartoi, T.; Graf, M.; Ong, H.; Ulbrich, M.H.; Masson, J.; et al. A CD36 ectodomain mediates insect pheromone detection via a putative tunnelling mechanism. Nat. Commun. 2016, 7, 11866.
- Fleischer, J.; Krieger, J. Insect pheromone receptors-Key elements in sensing intraspecific chemical signals. Front. Cell. Neurosci. 2018, 12, 425.
- Suh, G.S.; Wong, A.M.; Hergarden, A.C.; Wang, J.W.; Simon, A.F.; Benzer, S.; Axel, R.; Anderson, D.J. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 2004, 431, 854–859.
- Turner, S.L.; Ray, A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature 2009, 461, 277–281.
- Faucher, C.; Forstreuter, M.; Hilker, M.; de Bruyne, M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex, and olfactory context. J. Exp. Biol. 2006, 209, 2739–2748.
- Jones, W.D.; Cayirlioglu, P.; Kadow, I.G.; Vosshall, L.B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 2007, 445, 86–90.
- Kwon, J.Y.; Dahanukar, A.; Weiss, L.A.; Carlson, J.R. The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. USA 2007, 104, 3574–3578.
- Jones, W. Olfactory carbon dioxide detection by insects and other animals. Mol. Cells 2013, 35, 87–92.
- Badsha, F.; Kain, P.; Prabhakar, S.; Sundaram, S.; Padinjat, R.; Rodrigues, V.; Hasan, G. Mutants in Drosophila TRPC channels reduce olfactory sensitivity to carbon dioxide. PLoS ONE 2012, 7, e49848.
- Lin, H.; Chu, L.; Fu, T.; Dickson, B.J.; Chiang, A. Parallel neural pathways mediate CO2 avoidance responses in Drosophila. Science 2013, 340, 1338–1341.
- Wasserman, S.; Salomon, A.; Frye, M.A. Drosophila tracks carbon dioxide in flight. Curr. Biol. 2013, 23, 301–306.
- van Breugel, F.; Huda, A.; Dickinson, M.H. Distinct activity-gated pathways mediate attraction and aversion to CO2 in Drosophila. Nature 2018, 564, 420–424.
- Gibson, G.; Torr, S.J. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med. Vet. Entomol. 1999, 13, 2–23.
- Pickett, J.A.; Birkett, M.A.; Dewhirst, S.Y.; Logan, J.G.; Omolo, M.O.; Torto, B.; Pelletier, J.; Syed, Z.; Leal, W.S. Chemical ecology of animal and human pathogen vectors in a changing global climate. J. Chem. Ecol. 2010, 36, 113–121.
- Carey, A.F.; Carlson, J.R. Insect olfaction from model systems to disease control. Proc. Natl. Acad. Sci. USA 2011, 108, 12987–12995.
- Kepchia, D.; Moliver, S.; Chohan, K.; Phillips, C.; Luetje, C.W. Inhibition of insect olfactory behavior by an airborne antagonist of the insect odorant receptor co-receptor subunit. PLoS ONE 2017, 12, e0177454.
- Pham, K.C.; Ray, A. Conservation of olfactory avoidance in Drosophila species and identification of repellents for Drosophila suzukii. Sci. Rep. 2015, 5, 11527.
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In Focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351.
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing Drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402.
- Leal, W.S. The enigmatic reception of DEET—The gold standard of insect repellents. Curr. Opin. Insect Sci. 2014, 6, 93–98.
- DeGennaro, M.; McBride, C.; Seeholzer, L.; Nakagawa, T.; Dennis, J.E.; Goldman, C.; Jasinskiene, N.; James, A.A.; Vosshall, L.B. Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 2013, 498, 487–491.
- Ditzen, M.; Pellegrino, M.; Vosshall, L.B. Insect odorant receptors are molecular targets of the insect repellent DEET. Science 2008, 319, 1838–1842.
- Guo, H.; Kunwar, K.; Smith, D. Multiple channels of DEET repellency in Drosophila. Pest Manag. Sci. 2019, 76, 880–887.
- Dogan, E.B.; Ayres, J.W.; Rossignol, P.A. Behavioural mode of action of DEET: Inhibition of lactic acid attraction. Med. Vet. Entomol. 1999, 13, 97–100.
- Syed, Z.; Leal, W.S. Mosquitoes smell and avoid the insect repellent DEET. Proc. Natl. Acad. Sci. USA 2008, 105, 13598–13603.
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Martin, T. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 2016, 26, 127–142.
- DeGennaro, M. The mysterious multi-modal repellency of DEET. Fly 2015, 9, 45–51.
- Wang, Q.; Xu, P.; Andreazza, F.; Liu, Y.; Nomura, Y.; Duran, P.; Jiang, L.; Chen, M.; Takamatsu, G.; Ihara, M.; et al. Identification of multiple odorant receptors essential for pyrethrum repellency in Drosophila melanogaster. PLoS Genet. 2021, 17, e1009677.
- Stensmyr, M.C.; Dweck, H.K.; Farhan, A.; Ibba, I.; Strutz, A.; Mukunda, L.; Linz, J.; Grabe, V.; Steck, K.; Lavista-Llanos, S.; et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 2012, 151, 1345–1357.
- Becher, P.G.; Bengtsson, M.; Hansson, B.S.; Witzgall, P. Flying the fly: Long-range flight behavior of Drosophila melanogaster to attractive odors. J. Chem. Ecol. 2010, 36, 599–607.
- Kreher, S.A.; Mathew, D.; Kim, J.; Carlson, J.R. Translation of sensory input into behavioral output via an olfactory system. Neuron 2008, 59, 110–124.
- Wang, Q.; Xu, P.; Sanchez, S.; Duran, P.; Andreazza, F.; Isaacs, R.; Dong, K. Behavioral and physiological responses of Drosophila melanogaster and D. suzukii to volatiles from plant essential oils. Pest Manag. Sci. 2021, 77, 3698–3705.
More
