Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mihaela Carmen Eremia and Version 3 by Beatrix Zheng.
Polyhydroxyalkanoates (PHAs) are biodegradable and biocompatible biopolymers. These biomaterials have grown in importance in the fields of tissue engineering and tissue reconstruction for structural applications where tissue morphology is critical, such as bone, cartilage, blood vessels, and skin, among others. Furthermore, they can be used to accelerate the regeneration in combination with drugs, as drug delivery systems, thus reducing microbial infections. When cells are cultured under stress conditions, a wide variety of microorganisms produce them as a store of intracellular energy in the form of homo- and copolymers of [R]—hydroxyalkanoic acids, depending on the carbon source used for microorganism growth.
- polyhydroxyalkanoates
- microbial fermentation
- isolation
- purification
- characterization
1. Introduction
The currently increasing interest in polyhydroxyalkanoates (PHA) research for various applications [1] is due to their biodegradability [2][3][2,3], biocompatibility [4], bioresorbability [5] and piezoelectricity [1]. Furthermore, their various chemical properties have made them the topic of several scientific studies. As other biopolymers, they are environmentally friendly alternatives to non-biodegradable synthetic materials that have a negative impact on the environment [6]. PHAs are produced by a wide range of microorganisms under stress conditions of fermentation media composition, with a high concentration of carbon source, and the rest of the nutrients are present in limited quantities (nitrogen, phosphorus, potassium, magnesium or oxygen) [7][8][7,8]. Depending on the carbon source, the microorganisms make intracellular energy reserves under stress conditions in the form of homo- or copolymers of [R]-hydroxyalkanoic acids. PHAs have attracted interest as biodegradable polymers due to their biological (microbial) origin and non-toxic nature when compared to synthetic plastics, which can be highly toxic. The most-studied PHA is polyhydroxybutyrate (PHB) [9], and the most recently known is PHA, namely, polyhydroxyoctanoate (PHO) [10]. While PHB can be produced on an industrial scale, the production of mcl-PHA is still inferior to scl-PHA production due to the toxicity of the substrate. PHB has been studied in biomedical applications due to its thermoplastic behavior, suitable mechanical properties and versatile sintering methods [11]. Many studies have confirmed that mcl-PHA can be much more flexible and resistant than scl-PHA. These properties make it a good option for use in many fields, especially in the medical field or in obtaining films and coatings. PHA can be effective as a raw material in producing tablets, nanoparticles or drug scaffolds due to its pleasant physical properties and high biocompatibility [12]. PHA obtained under controlled conditions and with high purity can be used in tissue engineering through therapeutic applications such as vascular grafts, nerve tissue, or as a scaffold to promote cell growth by supplying nutrition [13][14].
2. Structure of Polyhydroxyalkanoates
Polyhydroxyalkanoates (Figure 1) make up a class of very versatile compounds[13, in which over 100 polymers have been shown to date14].Figure 1. Structure of polyhydroxyalkanoates (PHAs).
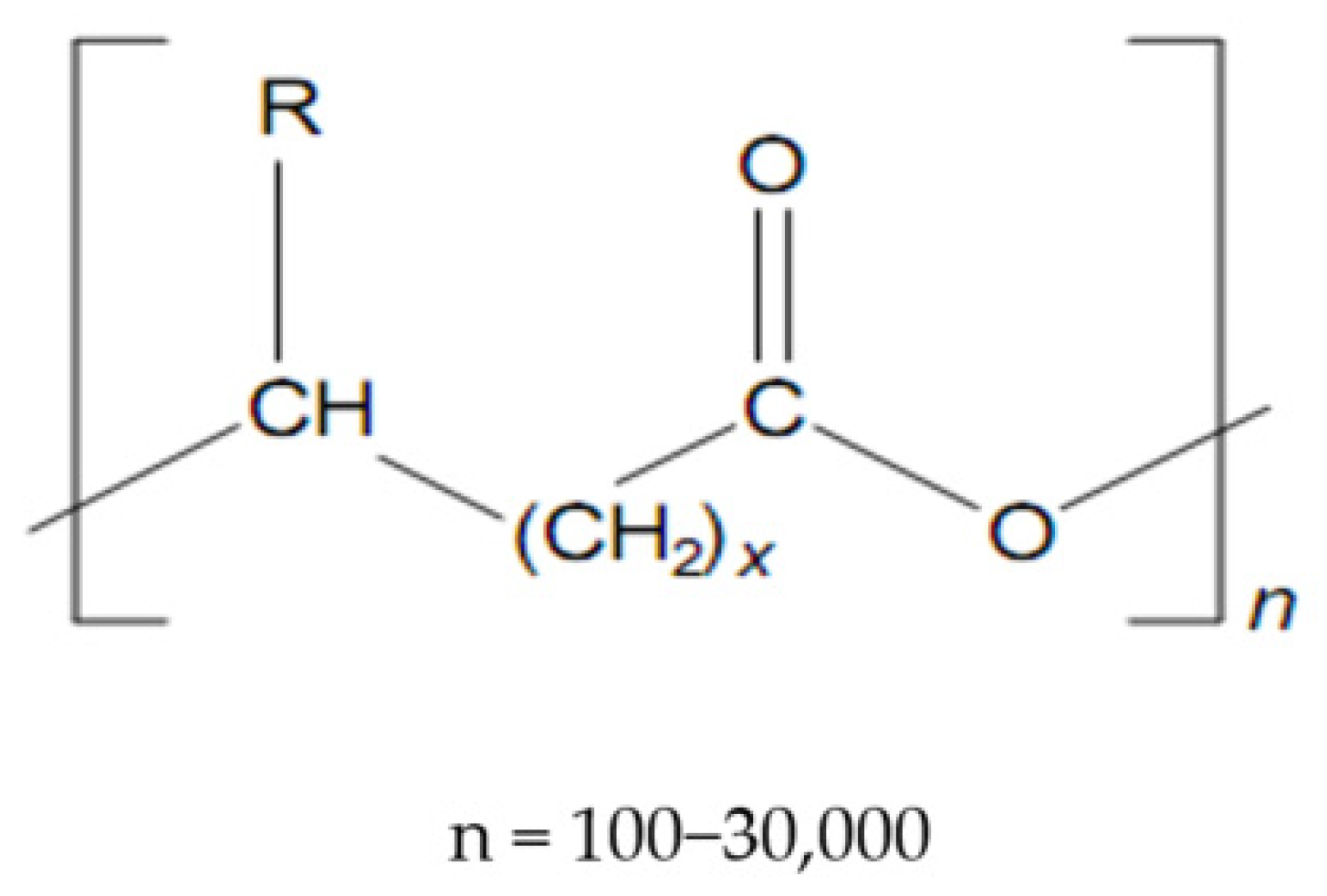
The PHA structure is differentiated according to two criteria:
- (a) The structure of the radicals attached to the carbon atoms with the R configuration in the skeleton of the polymer chain; these radicals represent the side chain of monomeric hydroxy acids;
- (b) The number and structure of the monomers in the polymer chain.
32. Brief Review of PHA Biomedical Applications
Among the various biomaterials available for tissue engineering and therapeutic applications, polyhydroxyalkanoates (PHAs) offer new properties as biomaterials of interest for medical applications due to their high biocompatibility and biodegradability and their various thermal-mechanical properties. The microbial polyesters poly 3-hydroxybutyrate (PHB), polyhydroxyvalerate (PHV) and poly [3-hydroxybutyrate 3-hydroxyvalerate] (PHBV) were most studied for orthopedic applications in recent decades as bone implants, which could form new bone in contact, without a chronic inflammatory response [11].
PHBV has been studied by numerous researchers [15][174], due to its biocompatibility with bone tissue. By degradation in vivo, PHBV forms D-3-hydroxybutyrate, which is normally found in human blood [16][175]. The biodegradability of PHA is the result of their stereo-specific structure with ester bonds, which can be enzymatically degraded in a biological medium. However, for various medical applications, polyesters need to be improved by functionalization [17][18][99,176].
The first commercial product to be approved by the FDA in 2007 was TephaFLEX from Tepha Medical Devices, a linear thermoplastic polyester produced by a recombinant E. coli fermentation process. This is an absorbable P (4HB) biopolymer, offering sutures that are 35% stronger than synthetic polydioxanone and 19% stronger than polypropylene [19][20][177,178]. Thus, P (4HB) can be transformed into a variety of absorbable medical devices, including sutures, patches, grafts, and textiles such as surgical meshes [21][179].
Phasix ™ mesh is a device made of P4HB [22][180]. It could become a treatment option for hernia because it has long-term mechanical strength and can prevent further postoperative complications [23][24][181,182]. Moreover, the P4HB biopolymer has been successfully implemented in tissue engineering. To increase the variety of uses for PHA-based biomaterials, researchers have investigated derivatization methods such as epoxidation, carboxylation, chlorination, hydroxylation, and pyrolysis [25][26][183,184]. Bioactivity, compatibility, biodegradability, hydrophobicity, moldability, and other qualities were improved [27][185]. Zibiao Li et al. recently investigated designed systems of PHA-based water-soluble polymers, functionalized PHAs with polar groups, or copolymerization of PHAs with hydrophilic components in a variety of polymeric designs [28][186]. They demonstrated that chemically modified water-soluble PHAs have a considerable impact on material construction and possess remarkable properties, resulting in suitable intelligent biomaterials [29][187].
Sutures, slings, stents, repair patches, cardiovascular patches, heart valves, orthopedic pins, adhesion barriers, cardiovascular tissue engineering devices, articular cartilage, nerve, tendon, guided tissue repair/regeneration devices, nerve guides, bone marrow scaffolding, and wound dressings are all made with improved PHAs [30][31][188,189].
To date, Phantom Fiber™ is marketed as suture (Tornier Co., Monsanto, MN, USA), MonoMax® as suture (Braun Surgical Co., Ctra. Rubí, Spain), BioFiber™ as scaffold (P4HB polymer) (Tornier Co.), GalaFLEX as mesh (Galatea Corp., Bromma, Sweden) and Tornier® as a surgical mesh (Tornier Co.) [32][190].
43. Conclusions
Polyhydroxyalkanoates (PHAs) production presents special features, different from other well-known microbial polymers (e.g., polysaccharides). One interesting property of their biological synthesis is the possible use of precursors to induce the biopolymer structure. However, the published results regarding the fermentation yields contain relatively low levels of final concentrations, possibly due to the stress conditions of media composition, which limit the bioprocess performance. New genetic engineered mutant strains, alternative substrates, mixed crops, fed-batch or continuous operation could overcome such restrictions.
Other challenges are the non-water solubility, intracellular character of the biopolymer, requesting complex, and difficult and costly steps of isolation and purification, especially for medical applications requiring purity (surgical reconstruction and tissue engineering, involving direct contact with blood). The hydrophobic character could be improved by hydrophilic functionalization, enlarging the area of applications.
Although only polyhydroxybutyrate have been FDA-approved for such medical applications to date [33][191], their proven favorable properties (immunologically inert, biocompatible, rapid tissue ingrowth, bioresorbable, slow biodegradable tissue scaffolds), as well as a large number of promising studies with other PHAs, justifies the trust in an optimistic outlook regarding the development of these biopolymers.
