Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 4 by Amina Yu.
The term microbiota refers to the entire population of microorganisms that colonizes a particular location and includes not just bacteria, but also other microbes such as fungi, archaea, viruses, and protozoans. Cows have bacteria inhabiting the uterus even before calving and establish a unique endometrial microbiome within 20 min of calving where the microbiome is similar between cows that develop metritis and cows without endometritis until at least the second day postpartum.
- genital
- microbiome
- health
- cattle
- pathogens
1. The Genital Tract Contains a Dynamic Microbiota
In cattle and sheep, Bacteroidetes, Fusobacteria, and Proteobacteria are the most common phyla in the genital tract [1]. At the genera level, Aggregatibacter spp., and Streptobacillus spp. were the most abundant. Interestingly, Lactobacillus spp., the predominate microbial populations in the human genital tract, were present in 80% and 90% of vaginal samples from ewes and cows, respectively [2][3][4]. In women, where the genital microbiome is intensively investigated, there are different vaginal microbial community state types (CSTs). The CSTs are defined by the dominant Lactobacillus species; CST I: Lactobacillus crispatus (L. crispatus), CST II: L. gasseri, CST III: L. iners, and CST V: L. jensenni. The CST IV population is defined by the absence of Lactobacilli species and the large diversity with strict and facultative anaerobic bacteria [3]. Such groupings, if present in cattle, may be indicative of bacterial profiles beneficial for fertility.
2. Significance of the Genital Microbiome
The reproductive tract microbiome of cattle is relatively under-explored, particularly in terms of the specific taxonomic classification and functional aspect of the microbiome, which are beneficial for the development of diagnostic methods, such as microbial biomarkers and dysbiosis indexes. The genital microbes in humans and animals have protective functions against major pathogens. In women, Lactobacilli produces lactic acid, which regulates vaginal pH and inhibits the proliferation of pathogens [5]. In turn, symbiotic bacteria utilize the secretions of the genital tract such as mucus sugar and proteins as a source of essential nutrients [6]. Lactic acid also induces acidification of milieu within the vagina, which interferes with intracellular functions, leading to microbial elimination [5]. Results from in vitro studies revealed that Chlamydia trachomatis is inactivated when there are normal concentrations of lactic acid [7], as are Neisseria gonorrhoeae and Escherichia coli (E. coli) [8][9]. One of the potential benefits of the commensal bacteria in women is the protection against human immunodeficiency virus type 1 (HIV-1). It seems that Lactobacilli in the vagina protect women from contracting HIV-1 during sexual intercourse. Lactic acid and the resultant biofilm limit the number of free virions and thereby reduce the shedding of HIV [10][11]. In vitro, HIV-1 is irreversibly inactivated when challenged with normal concentrations of lactic acid [12]. This may be the mechanism by which Lactobacilli confers vaginal protection against pathogens.
The genital microbiome is also implicated in the sociochemical signaling in different species through the production of pheromones [13][14][15]. Pheromones are produced by the microbiome by either direct production of pheromonal cues or through the fermentation hypothesis, by which microbes metabolize existing endogenous organic compounds to produce highly volatile compounds [16]. Results from a study in buffalo indicated there was a repetitive Flehmen response of males exposed to vaginal mucus from females at estrus [17]. Interestingly, the pheromones elaborated vary with the diversity of the genital microbiome [13][18].
3. Origin of the Genital Microbiome
The composition of the neonate microbiome is determined by the mode of birth and environment [19]. In humans, the general microbiome of the neonate delivered vaginally is similar to the maternal vaginal microbiome, dominated by Lactobacillus, Prevotella, or Sneathia spp. Neonates delivered through caesarean-section have a general microbiome similar to the maternal skin microbiome, mainly composed of Staphylococcus, Corynebacterium, and Propionibacterium spp. [20] (Figure 1). Therefore, initial exposure to the maternal microbiome influences an individual’s microbial load and diversity [20][21]. In cattle, microbes such Bacteroides and Enterobacteriaceae present in the genital tract causing reproductive diseases are thought to originate from the gut or the environment [22][23]. The results from some studies suggested that the vaginal microbiome originates from the gastrointestinal system [24], whereas others concluded that the vaginal microbial population includes methanogen species, and therefore the vaginal microbiome contributes to the establishment of the digestive tract microbiota [25]. The hematogenous route is also an important possible route for uterine contamination with pathogens [26].
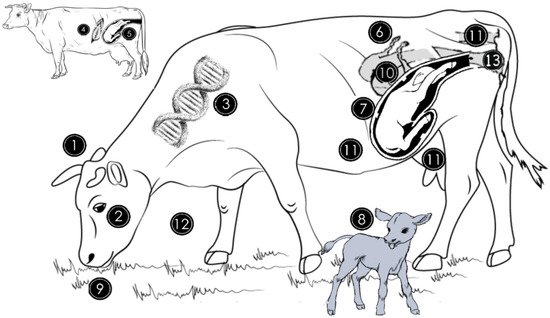
Figure 1. Origin of genital microbiome and factors that can affect the abundance and diversity of the microbial population. The genital microbiome is highly variable between species (1) and individuals of the same species (2). For instance, in cattle, genital microbiota is different between Gyr, Nellore, and Holstein breeds (3). The general microbiome of the newborn is similar to the mother’s skin microbiome if the delivery method was caesarean section (4) and is similar to the vaginal microbiome if it was natural delivery (5). Estrogen and progesterone hormone concentration variations during the estrous cycle influence bacterial growth in the genitalia by favoring some species at different times (6). During pregnancy, bacterial quantity and diversity decrease while archaeal abundance increases in vaginal milieu (7). Vaginal and uterine microbiomes of cows not diagnosed with metritis during the first month postpartum are similar in cows without uterine infections but differ from those with uterine infections (8). Dietary quality and quantity peripartum alter the endometrial microbiome through the provision of energy and protein nutrients (9). The uterine microbiome between 10 and 35 days postpartum is similar in cows not diagnosed with subclinical endometritis and those that will develop subclinical endometritis (10). Rumen, skin, rectum or feces (11) contribute to the establishment of the genital microbiome, while the environment (12) and intravaginal antibiotic therapy (13) can also alter the endometrial microbiota during a female’s lifetime.
4. Diversity of the Genital Microbiome
4.1. Vagina
Cows without uterine infections have in the vagina 15 taxa, predominantly Bacteroides (28.3%) and Enterobacteriaceae (17.8%), in addition to Victivallis (7.2%), Streptococcus (6.1%), Phyromonadaceae (5%), Alistipes (3.9%), Coriobacteriaceae (3.3%), Clostridium (3.3%), Betaproteobacteria (2.8%), Corynebacterineae (2.8%), Cytophagaceae (2.8%), Oscillibacter (2.8%), and Planctomycetaceae (2.8%) [27]. Cows with reproductive diseases such as purulent vaginal discharge have a more diverse vaginal microbiome containing 68 taxa, dominated by Bacteroides (35.83%), Enterobacteriaceae (18.62%), Histophilus (8.79%), Alistipes (4.34%), Flavobacteriaceae (1.77%), Victivallis (8.49%), Coriobacteriaceae (2.44%), Streptococcus (2.09%), Barnesiella (2.03%), and Oscillibacter (1.24%) [27]. Results from another study indicated that unclassified Enterobacteriaceae (21.05%), Ureaplasma (4.37%), and unclassified Bacteroidaceae (2.49%) were the most predominant [25]. At the phyla level, Tenericutes (36%), Proteobacteria (30%), Fusobacteria (7.6%), and Firmicutes (1.8%) were the most abundant [28]. In the study by Deng et al. (2019), Firmicutes (31.57%), Proteobacteria (24.08%), Bacteroidetes (12.96%), and Tenericutes (4.95%) were the most prevalent in the vagina. A comparison of the results from these two recent studies reveals that the proportions of the predominant microbial populations differ significantly between individuals.
4.2. Uterus
It is now known that cows have a natural microbiome in the uterus during the gestational period [29][30]. While the uterine microbes originate mainly from the vagina, and to a lesser extent, from the skin and gut, this microbiome is not as diverse as the vaginal microbiome [31]. Bacteria are always present in the uterus. F. necrophorum, Porphyromonas Levii, and T. pyogenes were detected in pregnant cows [29]. Interestingly, opportunistic microbes, such as Histophilus and Mycoplasmataceae, can become pathogenic [32][33]. Furthermore, the bacterial abundance in the uterus before calving is not associated with inflammation, which is indicative of a greater microbial tolerance during gestation [26].
5. Factors Affecting Genital Microbiome Diversity
The genital microbiome changes during the lifetime of females. Microbial populations in the reproductive tracts of animals are naturally selected because of different symbiotic functions. For example, in women, Lactobacilli use their small membrane extensions (for i.example., fimbriae) that adhere to the genital mucosa [34]. Likewise, the vaginal tissue is rich in collagen, a valuable source of nutrients for Aggregatibacter spp. [35][36]. There are also other factors that affect the genital microbial diversity with some being specific to the stage of the female reproductive cycle, and others are extrinsic such as nutrition. Interestingly, the vaginal microbiome could have originated from the intestinal microbiota from an evolutionary perspective because there are marked similarities between the microbial population of the two anatomical parts [24]. The thought was that this microbial similarity is due to the fact that the vagina and anus are juxtaposed, and feces are often in contact with vulva [24]. However, the prevailing thoughts at present are that the genital microbiome of the neonate initially originated from maternal tissue that is in contact with the neonate subsequent to parturition. The genital microbiome subsequently undergoes several changes during the lifetime of a female under the effect of the many factors, including the contamination by the microbiome of proximate organs such as the gastro-intestinal tract. Recent findings support the hypothesis (Figure 1) [25]. When there was a comparison of the changes in the microbial population in feces and vaginal samples collected before mating and at different stages of the gestational period, the fecal microbial diversity was the same, but the vaginal microbiome changed dynamically at different stages of the gestational period.
5.1. Intrinsic Factors
Individual variation in microbial species in the genital tract of bovids is thought to have effects of fertility outcomes [24]. This variation may explain how some animals develop resistance and others become infected with uterine diseases. Such differences are thought to be common in other mammals including humans (Figure 1) [37].
5.1.1. Species
The genital microbiome is diverse among animal species and also individual animals, which perturbs the regulation of reproductive hormones (Figure 1) [2]. For example, in two separate studies using either cows with endometritis or those administrated bacterial lipopolysaccharide (LPS), estradiol concentrations were lower, and there was a relatively prolonged period to the time of ovulation during the follicular phase of the reproductive cycle [38][39]. The vaginal microbiomes of cattle were composed of a large abundance of Enterococcus spp., Staphylococcus spp., and Streptococcus spp., which was different from the vaginal microbiome of ewes where there was a predominance of Bacillus spp., Corynebacterium spp., Escherichia spp., Staphylococcus spp., and Streptococcus spp. [40][41][42][43]. At the phyla level, the genital microbiomes in both cows and ewes were composed predominantly of Proteobacteria, Fusobacteria, and Bacteroidetes. At the genera level, Aggregatibacter spp. and Streptobacillus spp. were the predominant species [2]. While Lactobacilli are detected in 80% of ewes and 90% of cows, the total microbial population is less abundant, leading to the near-neutral vaginal environment compared to the acid environment in women where there is a large population of Lactobacilli [2]. In addition to Lactobacilli, cows and ewes share several genera, mainly Sneathia spp., Porphyromonas spp., and Prevotella spp. [3][2]. A small Firmicutes to Bacteroidetes ratio is an early indicator of cows that subsequently develop postpartum endometritis [44].
5.1.2. Breed (Genetic Background)
In Gyr cattle, a common dairy breed in South American countries such as Brazil, the vaginal microbiome is enriched with bacteria and fungi while there is a small population of archaea (Figure 1) [45]. Among bacteria, Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria were the most frequently detected. Mycosphaerella and Cladosporium were the most frequently detected fungal genera. While archaea were in low abundance, the Methanobrevibacter genus was the most abundant. In Nellore beef cattle, the vaginal microbiome is predominately composed of Firmicutes, Bacteroidetes, Proteobacteria, and up to 20% of unclassified bacteria [24]. Mycosphaerella was the most abundant fungal genus while Methanobrevibacter was the predominant archaeal genus. In Holstein Friesian cattle, the most ubiquitous dairy breed in North Africa, Europe, and the USA, the vaginal microbiome was predominately composed of Firmicutes, Tenericutes, Proteobacteria, and Bacteriodetes phyla. Other bacteria were detected in smaller quantities such as Actinobacteria and Spirochaetae [19].
5.1.3. Delivery Mode
The type of fetal delivery at parturition is one of the major factors affecting the genital microbiome diversity [20]. The general microbiome of the neonate is similar to the maternal skin microbiome if the parturition was by cesarean section and similar to the vaginal microbiome if it was natural delivery (Figure 1) [20]. Early established bacterial communities provide protection against pathogenic bacteria that may be infective agents to the neonate. While the maternal vagina is the main source for the natural microbiome, a unique neonatal microbiome is established shortly after parturition with microbes from other sources. For example, neonates delivered by caesarean section have a greater prevalence of methicillin-resistant Staphylococcus aureus (S. aureus) (MRSA) skin infections (64–82%) compared to when neonates are born without complications (i.e., natural delivery) [20].
5.1.4. Estrous Cyclicity
Estrous cycles in cattle are regulated by hormonal concentration changes with relatively higher concentrations of estradiol during proestrus and estrus and relatively higher progesterone concentrations during metestrus and diestrus, having marked effects on the vaginal pH in mammals [46][47]. Microbes are very sensitive to acidic milieu; therefore, it is thought that when there are greater estradiol concentrations, there will be effects on the microbiome in the genital milieu of some species, with effects varying as a result of estradiol concentrations (Figure 1). Results from a recent study indicated that Bacteroidete spp., Histophilus somni, Actinobacillus seminist, and unclassified Fusobacterium populations increase when there are relatively lower estradiol concentrations in the vaginal milieu [28]. When there are relatively higher estradiol concentrations in the vaginal milieu, unclassified Pasteurellaceae are the predominant microbes in the vagina. Likewise, when there are higher progesterone concentrations, the populations of microbes in the vagina are relatively larger [24][40].
5.1.5. Pregnancy
During the gestational period, there is lower microbial diversity of the vaginal microbiome and a greater archaeal population [24]. The bacterial species present in the vagina during the gestational period are less diverse due to the relatively higher progesterone concentrations that lead to suppression of the vaginal microbial population (Figure 1) [40]. The relatively lower microbial population in the vagina during the gestational period could lead to a greater risk of dysbiosis and abortion. Similarly, in humans, the microbial population in the vagina decreases as duration of the gestational period increases [48][49]. Unlike in humans, the vaginal microbiome of cattle is relatively stable throughout the gestational period [25]. In nonpregnant heifers, Pasteurellaceae spp. and Fusobacterium spp. were abundant in the vagina, whereas pregnant heifers had a greater prevalence of Pasteurella multocida [28].
5.1.6. Postpartum
Interestingly, the vaginal, and uterine microbiomes of cows without uterine inflammation during the first 50 DPP were similar and were highly enriched with Firmicutes [44]. This finding could be explained by the fact that soon after calving, the cervical lumen is less constricted, resulting in a mixing of the vaginal and uterine milieu with there being movement of these contents throughout the reproductive tract. It was hypothesized that in cows without uterine inflammation, the genital microbiome is not contaminated during calving by the external microbiome or at least not affected for an extended period subsequent to parturition. A comparison of the genital microbiome of pregnant cows before calving retrospectively with those that develop endometritis after 21 DPP to those that continue to have an uninfected uterus revealed that genital microbiome pre-calving was similar to the vaginal microbiome of healthy cows that did not develop uterine infections subsequent to 21 DPP [44].
5.2. Extrinsic Factors
5.2.1. Nutrition
The microbial population of the uterus of dairy cows during the postpartum period was reported to be affected by the nutrient content of the diet, especially the energy content, around the time of calving (Figure 1) [50]. Cows that were fed 80% of the energy requirement had uterine inflammation, and the predominant species of the uterine microbiome were Bacteroidetes and Fusobacteria. A comparison of the uterine microbiome pre-calving to that post-calving in cows that developed metritis with those that did not contract metritis indicated that cows with metritis had predominantly Bacteroides and Fusobacteria and a lower abundance of Proteobacteria and Tenericutes, which was markedly different from those that did not have metritis [26]. Therefore, nutrition affects the genital microbiome through the modulation of general metabolism and immune functions and therefore affects the occurrence of dysbiosis and genital infections.
5.2.2. Genital Pathologies
Metritis, which is indicated by a marked inflammation of the endometrium and myometrium soon after calving and before day 21 postpartum, is characterized by relatively lower abundance of the vaginal microbiome quantities and higher relative abundance of Bacteroides, Porphyromonas, and Fusobacterium [26][51]. Likewise, cows with metritis had a uterine microbiome that was markedly enriched with F. necrophorum, Porphyromonas levii, and Prevotella melaninogenica compared with cows without metritis [52]. E. coli is another interesting bacterium that is detected early postpartum in most of the cows because the presence of this microbe is important for the development of F. necrophorum, leading to metritis, while the latter bacterium is often detected in association with T. pyogenes in the case of endometritis [26][53]. In women with mild or moderate vaginal atrophy, the vaginal microbial population tends to be predominantly invasive pathogens, mainly Streptococcus and Prevotella [54]. Microbiomes in cattle diagnosed with necrotic vulvovaginitis (BNVV), as compared with those not diagnosed with BNVV, are characterized by a relatively microbial diversity and a large abundance of Bacateroidetes [55].
References
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey D. Swartz; Medora Lachman; Kelsey Westveer; Thomas O’Neill; Thomas Geary; Rodney W. Kott; James G. Berardinelli; Patrick G. Hatfield; Jennifer M. Thomson; Andrew Roberts; et al.Carl J. Yeoman Characterization of the Vaginal Microbiota of Ewes and Cows Reveals a Unique Microbiota with Low Levels of Lactobacilli and Near-Neutral pH. Frontiers in Veterinary Science 2014, 1, 19, 10.3389/fvets.2014.00019.
- Elizabeth A. Miller; Deanna E. Beasley; Robert R. Dunn; Elizabeth A. Archie; Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique?. Frontiers in Microbiology 2016, 7, 1936, 10.3389/fmicb.2016.01936.
- Wallace Jeng Yang Chee, Shu Yih Chew & Leslie Thian Lung Than; Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microbial Cell Factories 2020, 19, 203, https://doi.org/10.1186/s12934-020-01464-4.
- Deirdre E. O’Hanlon; Thomas R. Moench; Richard A. Cone; Vaginal pH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074, 10.1371/journal.pone.0080074.
- Gilda Tachedjian; Muriel Aldunate; Catronia S. Bradshaw; Richard A. Cone; The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Research in Microbiology 2017, 168, 782-792, 10.1016/j.resmic.2017.04.001.
- Paola Nardini; Rogers Alberto Nahui Palomino; Carola Parolin; Luca Laghi; Claudio Foschi; Roberto Cevenini; Beatrice Vitali; Antonella Marangoni; Lactobacillus crispatus inhibits the infectivity of Chlamydia trachomatis elementary bodies, in vitro study. Scientific Reports 2016, 6, 29024, 10.1038/srep29024.
- Michelle A Graver; Jeremy J Wade; The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Annals of Clinical Microbiology and Antimicrobials 2011, 10, 8-8, 10.1186/1476-0711-10-8.
- Erika V. Valore; Christina H. Park; Sorina L. Igreti; Tomas Ganz; Antimicrobial components of vaginal fluid. American Journal of Obstetrics and Gynecology 2002, 187, 561-568, 10.1067/mob.2002.125280.
- Taha E. Taha; Donald R. Hoover; Gina A. Dallabetta; Newton I. Kumwenda; Laban A. R. Mtimavalye; Li-Ping Yang; George N. Liomba; Robin L. Broadhead; John D. Chiphangwi; Paolo G. Miotti; et al. Bacterial vaginosis and disturbances of vaginal flora. AIDS 1998, 12, 1699-1706, 10.1097/00002030-199813000-00019.
- Thuy Hoang; Emily Toler; Kevin Delong; Nomfuneko A. Mafunda; Seth M. Bloom; Hannah C. Zierden; Thomas R. Moench; Jenell S. Coleman; Justin Hanes; Douglas S. Kwon; et al.Samuel K. LaiRichard A. ConeLaura M. Ensign The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis. PLoS Pathogens 2020, 16, e1008236, 10.1371/journal.ppat.1008236.
- Muriel Aldunate; David Tyssen; Adam Johnson; Tasnim Zakir; Secondo Sonza; Thomas Moench; Richard Cone; Gilda Tachedjian; Vaginal concentrations of lactic acid potently inactivate HIV. Journal of Antimicrobial Chemotherapy 2013, 68, 2015-2025, 10.1093/jac/dkt156.
- M. Srinivasan; M. Adnane; G. Archunan; Significance of cervico-vaginal microbes in bovine reproduction and pheromone production – A hypothetical review. Research in Veterinary Science 2021, 135, 66-71, 10.1016/j.rvsc.2021.01.003.
- Archunan, G.; Rajanarayanan, S.; Karthikeyan, K. Neurobiology of Chemical Communication; Carla Mucignat-Caretta, Eds.; CRC Press/Taylor & Francis LLC: Boca Raton, FL, USA,, 2014; pp. 461-479.
- G Archunan; Reproductive enhancement in buffalo: looking at urinary pheromones and hormones.. Iranian journal of veterinary research 2020, 21, 163-171.
- S. LeClaire; J. F. Nielsen; C. M. Drea; Bacterial communities in meerkat anal scent secretions vary with host sex, age, and group membership. Behavioral Ecology 2014, 25, 996-1004, 10.1093/beheco/aru074.
- R Sankar; G Archunan; Flehmen response in bull: role of vaginal mucus and other body fluids of bovine with special reference to estrus. Behavioural Processes 2004, 67, 81-86, 10.1016/j.beproc.2004.02.007.
- Sarah LeClaire; Staffan Jacob; Lydia K. Greene; George R. Dubay; Christine M. Drea; Social odours covary with bacterial community in the anal secretions of wild meerkats. Scientific Reports 2017, 7, 1-13, 10.1038/s41598-017-03356-x.
- Daniel Lazzari Quadros; Ricardo Zanella; Carlos Bondan; Giovana Ciacci Zanella; Fernanda Luiza Facioli; Arthur Nery da Silva; Eraldo Lourenso Zanella; Study of vaginal microbiota of Holstein cows submitted to an estrus synchronization protocol with the use of intravaginal progesterone device. Research in Veterinary Science 2020, 131, 1-6, 10.1016/j.rvsc.2020.03.027.
- Maria G. Dominguez-Bello; Elizabeth K. Costello; Monica Contreras; Magda Magris; Glida Hidalgo; Noah Fierer; Rob Knight; Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences 2010, 107, 11971-11975, 10.1073/pnas.1002601107.
- Sarkis K. Mazmanian; Cui Hua Liu; Arthur O. Tzianabos; Dennis L. Kasper; An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107-118, 10.1016/j.cell.2005.05.007.
- Hannah M. Wexler; Shyam Daya; Kenneth I. Berns; Bacteroides : the Good, the Bad, and the Nitty-Gritty. Clinical Microbiology Reviews 2007, 20, 593-621, 10.1128/cmr.00008-07.
- Nicole R. Kraipowich; David L. Morris; Gayle L. Thompson; Gary L. Mason; Bovine abortions associated with Bacteroides fragilis fetal infection.. Journal of Veterinary Diagnostic Investigation 2000, 12, 369-371, 10.1177/104063870001200413.
- Mateus Laguardia-Nascimento; Kelly Moreira Grillo Ribeiro Branco; Marcela Ribeiro Gasparini; Silvia Giannattasio-Ferraz; Laura Rabelo Leite; Flávio Marcos Gomes Araújo; Anna Christina De Matos Salim; Jacques Robert Nicoli; Guilherme Correa De Oliveira; Edel Barbosa-Stancioli; et al. Vaginal Microbiome Characterization of Nellore Cattle Using Metagenomic Analysis. PLoS ONE 2015, 10, e0143294, 10.1371/journal.pone.0143294.
- Feilong Deng; Maryanna McClure; Rick Rorie; Xiaofan Wang; Jianmin Chai; Xiaoyuan Wei; Songjia Lai; Jiangchao Zhao; The vaginal and fecal microbiomes are related to pregnancy status in beef heifers. Journal of Animal Science and Biotechnology 2019, 10, 1-13, 10.1186/s40104-019-0401-2.
- Klibs N. Galvão; Rodrigo C. Bicalho; Soo Jin Jeon; Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows. Journal of Dairy Science 2019, 102, 11786-11797, 10.3168/jds.2019-17106.
- N.F. Rodrigues; J. Kästle; Tarcisio José Domingos Coutinho; Aline Teixeira Amorim; G.B. Campos; V.M. Santos; L.M. Marques; J. Timenetsky; S.T. De Farias; Qualitative analysis of the vaginal microbiota of healthy cattle and cattle with genital-tract disease. Genetics and Molecular Research 2015, 14, 6518-6528, 10.4238/2015.june.12.4.
- Riley Messman; Zully E Contreras-Correa; Henry A Paz; George Perry; Caleb O Lemley; Vaginal bacterial community composition and concentrations of estradiol at the time of artificial insemination in Brangus heifers. Journal of Animal Science 2020, 98, 98, 10.1093/jas/skaa178.
- Cecilia Christensen Karstrup; Kirstine Klitgaard; Tim Kåre Jensen; Jørgen Steen Agerholm; Hanne Gervi Pedersen; Presence of bacteria in the endometrium and placentomes of pregnant cows. Theriogenology 2017, 99, 41-47, 10.1016/j.theriogenology.2017.05.013.
- Stephen Moore; A. C. Ericsson; S. E. Poock; P. Melendez; M. C. Lucy; Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. Journal of Dairy Science 2017, 100, 4953-4960, 10.3168/jds.2017-12592.
- I. Martin Sheldon; James G. Cronin; John J. Bromfield; Tolerance and Innate Immunity Shape the Development of Postpartum Uterine Disease and the Impact of Endometritis in Dairy Cattle. Annual Review of Animal Biosciences 2019, 7, 361-384, 10.1146/annurev-animal-020518-115227.
- Guda van der Burgt; Wendy Clark; Richard Knight; Cattle fertility problems and Histophilus somni. Veterinary Record 2007, 160, 600-600, 10.1136/vr.160.17.600.
- Nicholas, R.; Ayling, R.; McAuliffe. Mycoplasma Diseases of Ruminants; CAB International: Oxfordshire, UK, 2008; pp. 208-215.
- S. Kovachev; Defence factors of vaginal lactobacilli. Critical Reviews in Microbiology 2017, 44, 31-39, 10.1080/1040841x.2017.1306688.
- Gaoyan Tang; Todd Kitten; Cindy L. Munro; George C. Wellman; Keith P. Mintz; EmaA, a Potential Virulence Determinant of Aggregatibacter actinomycetemcomitans in Infective Endocarditis. Infection and Immunity 2008, 76, 2316-2324, 10.1128/iai.00021-08.
- Daniela Ulrich; Sharon L. Edwards; Vincent Letouzey; Kai Su; Jacinta F. White; Anna Rosamilia; Caroline E. Gargett; Jerome A. Werkmeister; Regional Variation in Tissue Composition and Biomechanical Properties of Postmenopausal Ovine and Human Vagina. PLoS ONE 2014, 9, e104972, 10.1371/journal.pone.0104972.
- The Human Microbiome Project Consortium; Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207-214, 10.1038/nature11234.
- I. Sheldon; D. Noakes; A. Rycroft; Dirk Pfeiffer; H Dobson; Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. REPRODUCTION 2002, 123, 837-845, 10.1530/reprod/123.6.837.
- Erin Williams; D.P. Fischer; D.E. Noakes; Gary England; A. Rycroft; H. Dobson; I.M. Sheldon; The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 2007, 68, 549-559, 10.1016/j.theriogenology.2007.04.056.
- C. Otero; C. Silva de Ruiz; R. Ibañez; O.R. Wilde; A.A.P. De Ruiz Holgado; M.E. Nader-Macias; Lactobacilli and Enterococci Isolated from the Bovine Vagina During the Estrous cycle. Anaerobe 1999, 5, 305-307, 10.1006/anae.1999.0245.
- C. Otero; L. Saavedra; C. Silva De Ruiz; O. Wilde; A.R. Holgado; M.E. Nader-Macias; Vaginal bacterial microflora modifications during the growth of healthy cows. Letters in Applied Microbiology 2000, 31, 251-254, 10.1046/j.1365-2672.2000.00809.x.
- G. J. Sawyer; OBSERVATIONS ON THE BACTERIAL POPULATION OF THE OS CERVIX OF THE EWE BEFORE AND AFTER EMBRYO DEATH. Australian Veterinary Journal 1977, 53, 542-544, 10.1111/j.1751-0813.1977.tb07942.x.
- J. Manes; M.A. Fiorentino; G. Kaiser; F. Hozbor; R. Alberio; E. Sanchez; F. Paolicchi; Changes in the aerobic vaginal flora after treatment with different intravaginal devices in ewes. Small Ruminant Research 2010, 94, 201-204, 10.1016/j.smallrumres.2010.07.021.
- Raúl Miranda-CasoLuengo; Junnan Lu; Erin J. Williams; Aleksandra A. Miranda-CasoLuengo; Stephen D. Carrington; Alexander C. O. Evans; Wim G. Meijer; Delayed differentiation of vaginal and uterine microbiomes in dairy cows developing postpartum endometritis. PLoS ONE 2019, 14, e0200974, 10.1371/journal.pone.0200974.
- Silvia Giannattasio-Ferraz; Mateus Laguardia-Nascimento; Marcela Ribeiro Gasparini; Laura Rabelo Leite; Flávio Marcos Gomes Araujo; Anna Christina De Matos Salim; André Penido De Oliveira; Jacques Robert Nicoli; Guilherme Corrêa De Oliveira; Flavio Guimarães Da Fonseca; et al.Edel Figueiredo Barbosa-Stancioli A common vaginal microbiota composition among breeds of Bos taurus indicus (Gyr and Nellore). Brazilian Journal of Microbiology 2019, 50, 1115-1124, 10.1007/s42770-019-00120-3.
- George I. Gorodeski; Ulrich Hopfer; Chung Chiun Liu; Ellen Margles; Estrogen Acidifies Vaginal pH by Up-Regulation of Proton Secretion via the Apical Membrane of Vaginal-Ectocervical Epithelial Cells. Endocrinology 2005, 146, 816-824, 10.1210/en.2004-1153.
- G.A. Perry; B.L. Perry; Effect of preovulatory concentrations of estradiol and initiation of standing estrus on uterine pH in beef cows. Domestic Animal Endocrinology 2008, 34, 333-338, 10.1016/j.domaniend.2007.09.003.
- Pawel Gajer; Rebecca M. Brotman; Guoyun Bai; Joyce Sakamoto; Ursel M. E. Schütte; Xue Zhong; Sara S. K. Koenig; Li Fu; Zhanshan (Sam) Ma; Xia Zhou; et al.Zaid AbdoLarry J. ForneyJacques Ravel Temporal Dynamics of the Human Vaginal Microbiota. Science Translational Medicine 2012, 4, 132ra52-132ra52, 10.1126/scitranslmed.3003605.
- Kjersti Aagaard; Kevin Riehle; Jun Ma; Nicola Segata; Toni-Ann Mistretta; Cristian Coarfa; Sabeen Raza; Sean Rosenbaum; Ignatia Van Den Veyver; Aleksandar Milosavljevic; et al.Dirk GeversCurtis HuttenhowerJoseph PetrosinoJames Versalovic A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466, 10.1371/journal.pone.0036466.
- Giulia Esposito; Emiliano Raffrenato; Somwe D Lukamba; Mounir Adnane; Pete C Irons; Paul Cormican; Taurai Tasara; Aspinas Chapwanya; Characterization of metabolic and inflammatory profiles of transition dairy cows fed an energy-restricted diet. Journal of Animal Science 2020, 98, 98, 10.1093/jas/skz391.
- I. Martin Sheldon; Erin Williams; Aleisha N.A. Miller; Deborah Mary Nash; Shan Herath; Uterine diseases in cattle after parturition. The Veterinary Journal 2008, 176, 115-121, 10.1016/j.tvjl.2007.12.031.
- Federico Cunha; Soo Jin Jeon; Rodolfo Daetz; Achilles Vieira Neto; Jimena Laporta; K. Casey Jeong; Anthony F. Barbet; Carlos A. Risco; Klibs N. Galvão; Quantifying known and emerging uterine pathogens, and evaluating their association with metritis and fever in dairy cows. Theriogenology 2018, 114, 25-33, 10.1016/j.theriogenology.2018.03.016.
- M.L.S. Bicalho; V.S. Machado; G. Oikonomou; Robert Gilbert; R.C. Bicalho; Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and uterine diseases of dairy cows. Veterinary Microbiology 2012, 157, 125-131, 10.1016/j.vetmic.2011.11.034.
- Rebecca M. Brotman; Michelle D. Shardell; Pawel Gajer; Doug Fadrosh; Kathryn Chang; Michelle I. Silver; Raphael P. Viscidi; Anne E. Burke; Jacques Ravel; Patti E. Gravitt; et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2018, 25, 1321-1330, 10.1097/gme.0000000000001236.
- N.Y. Shpigel; L. Adler-Ashkenazy; S. Scheinin; T. Goshen; A. Arazi; Z. Pasternak; Y. Gottlieb; Characterization and identification of microbial communities in bovine necrotic vulvovaginitis. The Veterinary Journal 2017, 219, 34-39, 10.1016/j.tvjl.2016.12.002.
More
