Although stem cells have been considered promising for the treatment of degenerative diseases by ‘seeding’ them into damaged tissues, it has recently been observed that the regenerative capacity of stem cells is influenced and regulated by the local immune response and in particular by macrophages, which constitute a central component of the damage response and are the coordinators of tissue repair and regeneration. Among the panoply of immune cells involved in the response to both acute and chronic wounds, recent discoveries have highlighted novel and often unexpected roles for certain types of immune cells in promoting a permissive local environment for effective cell replacement and restoration of tissue integrity. Some studies have shown that the control of inflammation is crucial in regenerative therapies: To be effective, regenerative therapies must block and control inflammation to allow tissue regeneration by resident stem cells. Indeed, the presence of inflammation inhibits the regenerative action of tissue-resident mesenchymal stem cells (MSCs). Recent papers suggest that an innovative regenerative strategy could be to polarize macrophages from the M1 inflammatory state to the M2 anti-inflammatory state utilizing immune cells. These reviews conclude that next-generation regenerative therapies need an immune-centric approach instead of the use of stem cells. Thus, depending on the tissue or organ targeted, regenerative strategies could be developed to stimulate macrophage polarization or to recruit subpopulations of pro-healing macrophages. Already, it has been observed that the regeneration of myocardial tissue after ischemia was induced by macrophages that regulate resident stem cells and promote regeneration, suggesting that targeting macrophages could be a new strategy to improve infarct healing and repair. The regenerative and stem-cell-controlling capacity of macrophages has also recently been demonstrated in bone tissue: mesenchymal stem cells act through a paracrine and immune-modulatory and non-differentiative mechanism and the microenvironment and immune system regulate the activity of MSCs regardless of the tissue from which they originate. Based on the role played by several types of macrophages and lymphocytes in the wound-healing response, it is tempting to hypothesize that interventions that reduce the M1 macrophage phenotype and promote M2 may represent a new therapy to induce tissue regeneration.
- wound healing
- diabetic foot
- immune system
- monocytes
- lymphocytes
- immune centric revolution
1. How to Switch to M2 Regenerative Phenotype?
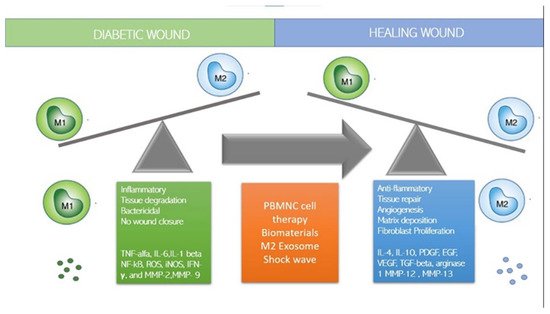
2. Immune-Cell-Based Cell Therapy
| Description | Result | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Zuloff-Shani et al. 2004 | Treatment of chronic ulcers with blood-derived macrophages activated by hypo-osmotic shock in over 1000 patients | Reduction of the healing time, reduction of risk of complications and morbidity. Improvement of the quality of life for long-suffering patients | [23] | ||||
| Danon et al. | Decubital ulcers of 72 patients (average age 82), were treated by local injection of macrophages prepared from a blood unit in a closed sterile system. The remaining 127 patients (average age 79) were treated conventionally and served as controls. No exclusion criteria were applied. | In the macrophage-treated group, 27% healed, while only 6% healed in the control group (p < 0.001). Moreover, the macrophage-treated group showed a faster healing (p < 0.02) | [24] | ||||
| Zuloff-Shani et al. 2010 | 100 consecutive elderly patients with a total of 216 stage III or IV pressure ulcers, 66 patients were assigned to the autologous macrophages group, 38 patients were assigned to the standard care treatments (38 patients.) | Percentage of completely closed wounds (wound level and patient level) were significantly better (p < 0.001/p < 0.001, respectively) in all patients in favor of AMS, as well as in the subset of diabetic patients (p < 0.001/p < 0.001). | [25] | ||||
| Moriya, J et al. | Retrospective study on 42 patients with severe intermittent claudication, ischemic rest pain, or non-healing ischemic ulcers caused by peripheral arterial disease, including thromboangiitis obliterans, and who had not responded to conventional therapy that included nonsurgical and surgical revascularization (no option). | Improvement of ischemic symptoms was observed in 60% to 70% of the patients. The annual rate of major amputation was decreased significantly by treatment. The survival rate of younger responders was better than that of non-responders. | Lyophilized, decellularized porcine small intestine submucosa (SIS). Matrix is derived from a single layer of porcine small intestinal submucosa (SIS) technology. The technology provides an intact three-dimensional extracellular matrix which allows for host cell migration. The SIS is freeze-dried and sterilized with ethylne oxide gas in preparation for clinical use |
Porcine small intestine submucosa (SIS). No cross-link[26] |
|||
| [ | 62 | ] | Huang, P.P et al. | 150 patients with peripheral arterial disease were randomised to mobilized PBMNC 76 cases or BMMNC 74 cases implanted, follow up for 12 weeks. Primary outcomes were safety and efficacy of treatment, based on ankle-brachial index (ABI) and rest pain | |||
| Allomend | Decellularized donated human dermal tissue, | Significant improvement of the ABI, skin temperature and rest pain was observed in both groups after transplantation and was better I PBMNC group. However, there was no significant difference between two groups for pain-free walking distance, transcutaneous oxygen pressure, ulcers, and rate of lower limb amputation | with significant removal of cellular debris (including DNA and RNA), proteins and antigens. The process does not require the use of detergents or enzymes, thereby mitigating the possibility of harmful residuals in the tissue. The decellularization process also inactivates microorganisms through cellular disruption. USA only, not available in Europe[27] |
||||
| Human dermal tissue | No cross link | [61][62][63 | Liotta, F et al. | Autologous Non-Mobilized Enriched Circulating Endothelial Progenitors obtained from non-mobilized peripheral blood by immunomagnetic selection of CD14+ and CD34+ cells) or BM-MNC were injected into the gastrocnemius of the affected limb in 23 and 17 patients with no option critical limb ischemia. | After 2 yrs follow-up, both groups showed significant and progressive improvement in muscle perfusion (primary endpoint), rest pain, consumption of analgesics, pain-free walking distance, wound healing, quality of life, ankle-brachial index, toe-brachial index, and transcutaneous PO2 | [28] | |
| Dubsky, M et al. | 28 patients with diabetic foot disease (17 treated by bone marrow cells and 11 by peripheral blood mononuclear cell) were included into an active group and 22 patients into a control group without cell treatment. | The transcutaneous oxygen pressure increased significantly (p < 0.05) compared with baseline in both active groups after 6 months, with no significant differences between bone marrow cells and peripheral blood cell groups, while no change in the control group was observed. The rate of major amputation by 6 months was significantly lower in the active cell therapy group compared with that in the control group (11.1% vs. 50%, p = 0.0032), with no difference between bone marrow cells and peripheral blood cells. | [29] | ||||
| ] | |||||||
| DermaMatrix | Cadaveric human allograft treated with a disinfectant solution that combines detergents with acidic and antiseptic reagents. USA only, not available in Europe |
Human dermal tissue No cross link |
[61][62][63] | Persiani, F. et al. | |||
| Dermacell | Decellularized regenerative human tissue matrix allograft processed using proprietary technology that removes at least 97% of donor DNA without compromising the desired biomechanical structure or biochemical properties. USA only, not available in Europe |
Human dermal tissue No cross link |
[61][ | 50 diabetic patients affected by CLI underwent PBMNCs implant (32 patients underwent PBMNCs therapy associated with endovascular revascularization, 18 patients, non-option CLI) | The follow-up period was 10 months. In the PBMNC group + revascularization TcPO, pain VAS Scale improved. In PBMNCs therapy group, the mean TcPO2 improved from 16.2 ± 7.2 mmHg to 23.5 ± 8.4 mmHg (p < 0.001), and VAS score means decreased from 9 ± 1.1 to 4.1 ± 3.3 (p < 0.001). Major amputation was observed in 3 cases (9.4%), both in adjuvant therapy group and in PBMNCs therapy. (16.7%) (P ¼ 0.6) as the therapeutic choice (PBMNCs therapy group). |
[30] | |
| De Angelis, B et al. | Prospective, not randomized study based on a treated group who did not respond to conventional therapy (n = 43) when implanted with A-PBMNC cells versus a historically matched control group. Patients of both groups were suffering from CLI Fontaine scale IV with chronic ulcers |
The A-PBMNC-treated group showed a statistically significant improvement of limb rescue of 95.3% versus 52.2% of the control group (p < 0.001) at 2 years. The A-PBMNC group also showed reduction in pain at rest, increased maximum walking distance, and healing of the wound and an overall improvement in the quality of life. Post-treatment radiological studies showed an improvement of vascularization with the formation of new collateral and by histological findings. | [31] | ||||
| Dubsky et al. | 31 patients with DFU and CLI treated by autologous stem cells and 30 patients treated by PTA were included in the study; 23 patients with the same inclusion criteria who could not undergo PTA or cell therapy formed the control group. |
Amputation-free survival after 6 and 12 months was significantly greater in the cell therapy and PTA groups compared with controls (p < 0.001 and p < 0.0029, respectively) without significant differences between the active treatment groups. Increase in TcPO2 did not differ between cell therapy and PTA groups until 12 months but TcPO2 in the control group did not change over the follow-up period. More healed ulcers were observed up to 12 months in the cell therapy group compared with the PTA and control groups (84% vs. 57.7% vs. 44.4%; p < 0.042). |
[32] | ||||
| Scatena et al. | The study included 76 NO-CLI patients with DFUs. All patients were treated with the same standard care (control group), but 38 patients were also treated with autologous PBMNC implants. |
Only 4 out 38 amputations (10.5%) were observed in the PBMNC group, while 15 out of 38 amputations (39.5%) were recorded in the control group (p = 0.0037). The Kaplan–Meier curves and the log-rank test results showed a significantly lower amputation rate in the PBMNCs group vs. the control group (p = 0.000). At two years follow-up, nearly 80% of the PBMNCs group was still alive vs. only 20% of the control group (p = 0.000). In the PBMNC group, 33 patients healed (86.6%) while only one patient healed in the control group (p = 0.000). |
[33] | ||||
| Di Vieste et al. | Case report of a 59-year-old patient with type 2 diabetes mellitus who had a gangrene of the right toe. After an ineffective angioplasty, it was decided to use a PBMNC therapy. | The patient underwent to amputation of the first necrotic toe and three PBMNC treatment sessions with complete surgical wound healing and limb rescue |
3. Mesenchymal Stem Cells (MSC)
4. Extracellular Vesicles (EVs) and Exosome (Exo)
5. Dermal Substitutes
| Primary Material Composition |
Source and Other Components | Refs. | |
|---|---|---|---|
| Nevelia | Porous resorbable double layer matrix 2 mm thickness made of stabilized native collagen type I and a silicone sheet 200 mm thickness mechanically reinforced with a polyester fabric. The extraction procedure and the freeze-drying process allow the structuring of the collagen into a matrix with optimal hydrophilicity, pore structure and pore size (20–125 μm) |
Bovine, Native collagen Type I. No glycosaminoglycan (GAG) added to improve cell attachment and proliferation. Glutaraldehyde Cross-linking |
[58][59][60] |
| Integra | Bilayer system for skin replacement made of a porous matrix of fibers of cross-linked bovine tendon collagen and glycosaminoglycan (chondroitin-6-sulfate) that is manufactured with a controlled porosity and defined degradation rate. The Integra pore size of 20 to 125 μm allows influx of cells. |
Bovine Tendon Type I Collage Shark cartilage -derived chondroitin-6-sulphate (GAG). Glutaraldehyde Cross-linking |
[61][62][63] |
| PriMatrix | Acellular dermal tissue matrix. comprising of both type I and type III collagen derived from fetal bovine dermis. This matrix is processed in a way to maintains the extracellular matrix in its native and undamaged state while removing all lipids, fats, cells, carbohydrates and non-collagenous proteins. |
Fetal Bovine collagen type I and type III collagen. No cross-link |
[62] |
| Oasis Wound Matrix |
|||
| 62 | |||
| ] | |||
| [ | 64 | ] |
References
- Zuloff-Shani, A.; Kachel, E.; Frenkel, O.; Orenstein, A.; Shinar, E.; Danon, D. Macrophage suspensions prepared from a blood unit for treatment of refractory human ulcers. Transfus. Apher. Sci. 2004, 30, 163–167.
- Danon, D.; Madjar, J.; Edinov, E.; Knyszynski, A.; Brill, S.; Diamantshtein, L.; Shinar, E. Treatment of human ulcers by application of macrophages prepared from a blood unit. Exp. Gerontol. 1997, 32, 633–641.
- Zuloff-Shani, A.; Adunsky, A.; Even-Zahav, A.; Semo, H.; Orenstein, A.; Tamir, J.; Regev, E.; Shinar, E.; Danon, D. Hard to heal pressure ulcers (stage III–IV): Efficacy of injected activated macrophage suspension (AMS) as compared with standard of care (SOC) treatment controlled trial. Arch. Gerontol. Geriatr. 2010, 51, 268–272.
- Moriya, J.; Minamino, T.; Tateno, K.; Shimizu, N.; Kuwabara, Y.; Sato, Y.; Saito, Y.; Komuro, I. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ. Cardiovasc. Interv. 2009, 2, 245–254.
- Huang, P.P.; Yang, X.F.; Li, S.Z.; Wen, J.C.; Zhang, Y.; Han, Z.C. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb. Haemost. 2007, 98, 1335–1342.
- Liotta, F.; Annunziato, F.; Castellani, S.; Boddi, M.; Alterini, B.; Castellini, G.; Mazzanti, B.; Cosmi, L.; Acquafresca, M.; Bartalesi, F.; et al. Therapeutic Efficacy of Autologous Non-Mobilized Enriched Circulating Endothelial Progenitors in Patients with Critical Limb Ischemia―The SCELTA Trial. Circ. J. 2018, 82, 1688–1698.
- Dubsky, M.; Jirkovska, A.; Bem, R.; Fejfarova, V.; Pagacova, L.; Sixta, B.; Varga, M.; Langkramer, S.; Sykova, E.; Jude, E.B. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab. Res. Rev. 2013, 29, 369–376.
- Persiani, F.; Paolini, A.; Camilli, D.; Mascellari, L.; Platone, A.; Magenta, A.; Furgiuele, S. Peripheral Blood Mononuclear Cells Therapy for Treatment of Lower Limb Ischemia in Diabetic Patients: A Single-Center Experience. Ann. Vasc. Surg. 2018, 53, 190–196.
- De Angelis, B.; Gentile, P.; Orlandi, F.; Bocchini, I.; Di Pasquali, C.; Agovino, A.; Gizzi, C.; Patrizi, F.; Scioli, M.G.; Orlandi, A.; et al. Limb Rescue: A New Autologous-Peripheral Blood Mononuclear Cells Technology in Critical Limb Ischemia and Chronic Ulcers. Tissue Eng. Part C Methods 2015, 21, 423–435.
- Dubský, M.; Jirkovská, A.; Bem, R.; Fejfarová, V.; Pagacová, L.; Nemcová, A.; Sixta, B.; Chlupac, J.; Peregrin, J.H.; Syková, E.; et al. Comparison of the effect of stem cell therapy and percutaneous transluminal angioplasty on diabetic foot disease in patients with critical limb ischemia. Cytotherapy 2014, 16, 1733–1738.
- Scatena, A.; Petruzzi, P.; Maioli, F.; Lucaroni, F.; Ventoruzzo, G.; Bolognese, L.; Ercolini, L. Does Autologous Peripheral Mononuclear Cells Implant Allow Foot Surgery in Diabetic Patients with Critical Limb Ischaemia not Eligible for Revascularization? In Proceedings of the 8th International Symposium Diabetic Foot, IDF, The Hague, The Netherlands, 22–25 May 2019; p. 95.
- Paige, J.T.; Kremer, M.; Landry, J.; Hatfield, S.A.; Wathieu, D.; Brug, A.; Lightell, D.J.; Spiller, K.L.; Woods, T.C. Modulation of inflammation in wounds of diabetic patients treated with porcine urinary bladder matrix. Regen. Med. 2019, 14, 269–277.
- Montanaro, M.; Meloni, M.; Anemona, L.; Servadei, F.; Smirnov, A.; Candi, E. Macrophage Activation and M2 Polarization in Wound Bed of Diabetic Patients Treated by Dermal/Epidermal Substitute Nevelia. Int. J. Low. Extrem. Wounds, 2020; in press.
- Uccioli, L.; Meloni, M.; Izzo, V.; Giurato, L. Use of Nevelia Dermal-Epidermal Regenerative Template in the Management of Ischemic Diabetic Foot Postsurgical Wounds. Int. J. Low. Extrem. Wounds 2020, 19, 282–288.
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 29.
- Varela, P.; Sartori, S.; Viebahn, R.; Salber, J.; Ciardelli, G. Macrophage immunomodulation: An indispensable tool to evaluate the performance of wound dressing biomaterials. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019830355.
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Liu, Z.; Huang, H. Immune-enhancing activities of chondroitin sulfate in murine macrophage RAW 264.7 cells. Carbohydr. Polym. 2018, 198, 611–619.
- Yin, Y.; He, X.T.; Wang, J.; Wu, R.X.; Xu, X.Y.; Hong, Y.L.; Tian, B.M.; Chen, F.M. Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl. Mater. Today 2020, 18, 100466.
- Di Vieste, G.; Formenti, I.; Balduzzi, G.; Masserini, B.; De Giglio, R. Use of autologous peripheral blood mononuclear cells in a case of diabetic foot. J. AMD 2019, 22, 230–233.
- Di Pardo, A.; Cappello, E.; Pepe, G.; Marracino, F.; Carrieri, V.; Maglione, V.; Pompeo, F. Infusion of autologous-peripheral blood mononuclear cells: A new approach for limb salvage in patients with diabetes. In Proceedings of the 7th International Diabetic Foot Congress Abu Dhabi, IFD Congress, Abu Dhabi, United Arab Emirates, 4–8 December 2017.
- Jetten, N.; Roumans, N.; Gijbels, M.J.; Romano, A.; Post, M.J.; de Winther, M.P.J.; Van Der Hulst, R.R.W.J.; Xanthoulea, S. Wound Administration of M2-Polarized Macrophages Does Not Improve Murine Cutaneous Healing Responses. PLoS ONE 2014, 9, e102994.
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.J.; Donners, M.M.P.C. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118.
- Saldaña, L.; Bensiamar, F.; Vallés, G.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res. Ther. 2019, 10, 58.
- Anton, K.; Banerjee, D.; Glod, J. Macrophage-Associated Mesenchymal Stem Cells Assume an Activated, Migratory, Pro-Inflammatory Phenotype with Increased IL-6 and CXCL10 Secretion. PLoS ONE 2012, 7, e35036.
- Saldaña, L.; Vallés, G.; Bensiamar, F.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Paracrine interactions between mesenchymal stem cells and macrophages are regulated by 1,25-dihydroxyvitamin D3. Sci. Rep. 2017, 7, 14618.
- Harrell, C.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467.
- Harrell, C.R.; Djonov, V.; Volarevic, V. The cross-talk between mesenchymal stem cells and immune cells in tissue repair and regeneration. Int. J. Mol. Sci. 2021, 22, 2472.
- Zhou, Y.; Singh, A.K.; Hoyt, R.F.; Wang, S.; Yu, Z.; Hunt, T.; Kindzelski, B.; Corcoran, P.C.; Mohiuddin, M.M.; Horvath, K.A. Regulatory T cells enhance mesenchymal stem cell survival and proliferation following autologous cotransplantation in ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2014, 148, 1131–1137.
- Faulknor, R.A.; Olekson, M.A.; Ekwueme, E.C.; Krzyszczyk, P.; Freeman, J.W.; Berthiaume, F. Hypoxia impairs mesenchymal stromal cell-induced macrophage M1 to M2 transition. Technology 2017, 5, 81–86.
- Inoue, O.; Usui, S.; Takashima, S.; Nomura, A.; Yamaguchi, K.; Takeda, Y.; Goten, C.; Hamaoka, T.; Ootsuji, H.; Murai, H.; et al. Diabetes impairs the angiogenic capacity of human adipose-derived stem cells by reducing the CD271+ subpopulation in adipose tissue. Biochem. Biophys. Res. Commun. 2019, 517, 369–375.
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553.
- Spiller, K.L.; Koh, T.J. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 2017, 122, 74–83.
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Chasing the recipe for a pro-regenerative immune system. Semin. Cell Dev. Biol. 2017, 61, 71–79.
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. 2018, 14, 337–345.
- Fadini, G.P.; Spinetti, G.; Santopaolo, M.; Madeddu, P. Impaired Regeneration Contributes to Poor Outcomes in Diabetic Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 34–44.
- Santopaolo, M.; Sambataro, M.; Spinetti, G.; Madeddu, P. Bone marrow as a target and accomplice of vascular complications in diabetes. Diabetes Metab. Res. Rev. 2020, 36, e3240.
- Wang, J.; Xia, J.; Huang, R.; Hu, Y.; Fan, J.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles alter disease outcomes via endorsement of macrophage polarization. Stem Cell Res. Ther. 2020, 11, 424.
- Heo, J.S.; Choi, Y.; Kim, H.O.; Matta, C. Adipose-Derived Mesenchymal Stem Cells Promote M2 Macrophage Phenotype through Exosomes. Stem Cells Int. 2019, 2019, 7921760.
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B.; Bolontrade, M.F. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708.
- Li, M.; Wang, T.; Tian, H.; Wei, G.; Zhao, L.; Shi, Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3793–3803.
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513.
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 29.
- Varela, P.; Sartori, S.; Viebahn, R.; Salber, J.; Ciardelli, G. Macrophage immunomodulation: An indispensable tool to evaluate the performance of wound dressing biomaterials. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019830355.
- Whiterel, C.E.; Graney, P.L.; Freytes, D.O.; Weingarten, M.S.; Spiller, K.L. Response of human macrophages to wound matrices in vitro. Wound Repair Regen. 2016, 24, 514–524.
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655.
- Tylek, T.; Blum, C.; Hrynevich, A.; Schlegelmilch, K.; Schilling, T.; Dalton, P.D.; Groll, J. Precisely defined fiber scaffolds with 40 μm porosity induce elongation driven M2-like polarization of human macrophages. Biofabrication 2020, 12, 025007.
- Yin, Y.; He, X.T.; Wang, J.; Wu, R.X.; Xu, X.Y.; Hong, Y.L.; Tian, B.M.; Chen, F.M. Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl. Mater. Today 2020, 18, 100466.
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Liu, Z.; Huang, H. Immune-enhancing activities of chondroitin sulfate in murine macrophage RAW 264.7 cells. Carbohydr. Polym. 2018, 198, 611–619.
- Agrawal, H.; Tholpady, S.S.; Capito, A.E.; Drake, D.B.; Katz, A.J. Macrophage phenotypes correspond with remodeling outcomes of various acellular dermal matrices. Open J. Regen. Med. 2012, 1, 51–59.
- Paige, J.T.; Kremer, M.; Landry, J.; Hatfield, S.A.; Wathieu, D.; Brug, A.; Lightell, D.J.; Spiller, K.L.; Woods, T.C. Modulation of inflammation in wounds of diabetic patients treated with porcine urinary bladder matrix. Regen. Med. 2019, 14, 269–277.
- Montanaro, M.; Meloni, M.; Anemona, L.; Servadei, F.; Smirnov, A.; Candi, E. Macrophage Activation and M2 Polarization in Wound Bed of Diabetic Patients Treated by Dermal/Epidermal Substitute Nevelia. Int. J. Low. Extrem. Wounds, 2020; in press.
- Uccioli, L.; Meloni, M.; Izzo, V.; Giurato, L. Use of Nevelia Dermal-Epidermal Regenerative Template in the Management of Ischemic Diabetic Foot Postsurgical Wounds. Int. J. Low. Extrem. Wounds 2020, 19, 282–288.
- Swartzwelter, B.J.; Verde, A.; Rehak, L.; Madej, M.; Puntes, V.F.; De Luca, A.C.; Boraschi, D.; Italiani, P. Interaction between macrophages and nanoparticles: In vitro 3d cultures for the realistic assessment of inflammatory activation and modulation of innate memory. Nanomaterials 2021, 11, 207.
