Bacteria and fungi are known to be inactivated not only by ultraviolet radiation but also by visible light. Viruses appear to be sensitive to visible (violet/blue) light.
- enveloped virus
- non-enveloped virus
- coronavirus
- photoinactivation
- riboflavin
- media
1. Introduction
Conventional disinfection techniques such as chemical disinfection or disinfection with ultraviolet radiation are very effective [2[1][2][3][4][5],3,4,5,6], but are not applicable in many everyday situations because, for example, ultraviolet radiation damages not only viruses but also human cells and various materials.
Blue and violet light are especially able to inactivate pathogenic bacteria and fungi [7,8,9,10,11,12,13,14,15][6][7][8][9][10][11][12][13][14] if the applied irradiation doses are high enough. Endogenous photosensitizers, such as porphyrins or flavins, naturally present in bacteria and fungi, absorb this visible light and subsequently generate reactive oxygen species such as 1O2, OH or H2O2. These intracellular reactive oxygen species attack DNA, proteins or membranes and, if the produced damage becomes too great, the cell dies. Human cells also contain such photosensitizers, but they have nevertheless proven to be very resistant to visible light [16,17,18,19,20,21,22,23,24][15][16][17][18][19][20][21][22][23].
2. Virus Experiments Performed in Liquids
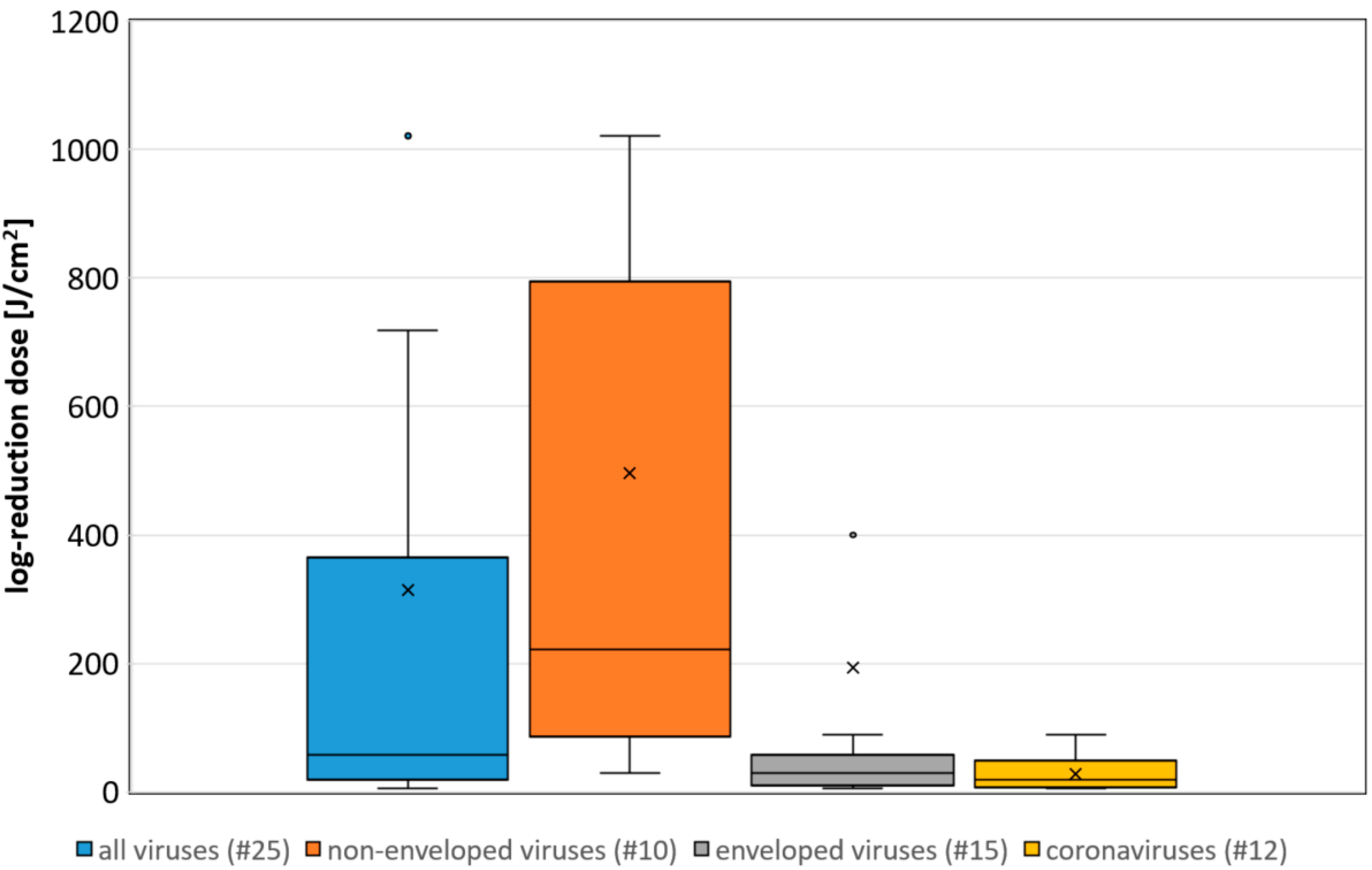
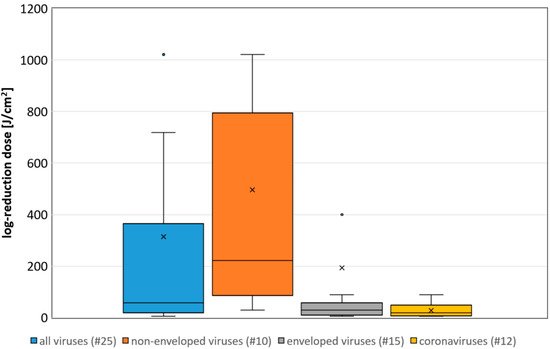 Figure 1. Boxplot of the determined necessary log-reduction dose for all, non-enveloped, enveloped and coronaviruses. Illustrated are the medians, and the quantiles of the result distribution. The number in brackets give the quantity of data sets for each virus category (not all outliers are depicted).
Figure 1. Boxplot of the determined necessary log-reduction dose for all, non-enveloped, enveloped and coronaviruses. Illustrated are the medians, and the quantiles of the result distribution. The number in brackets give the quantity of data sets for each virus category (not all outliers are depicted).3. A correlation between the Assumed Riboflavin Concentration and the Sensitivity
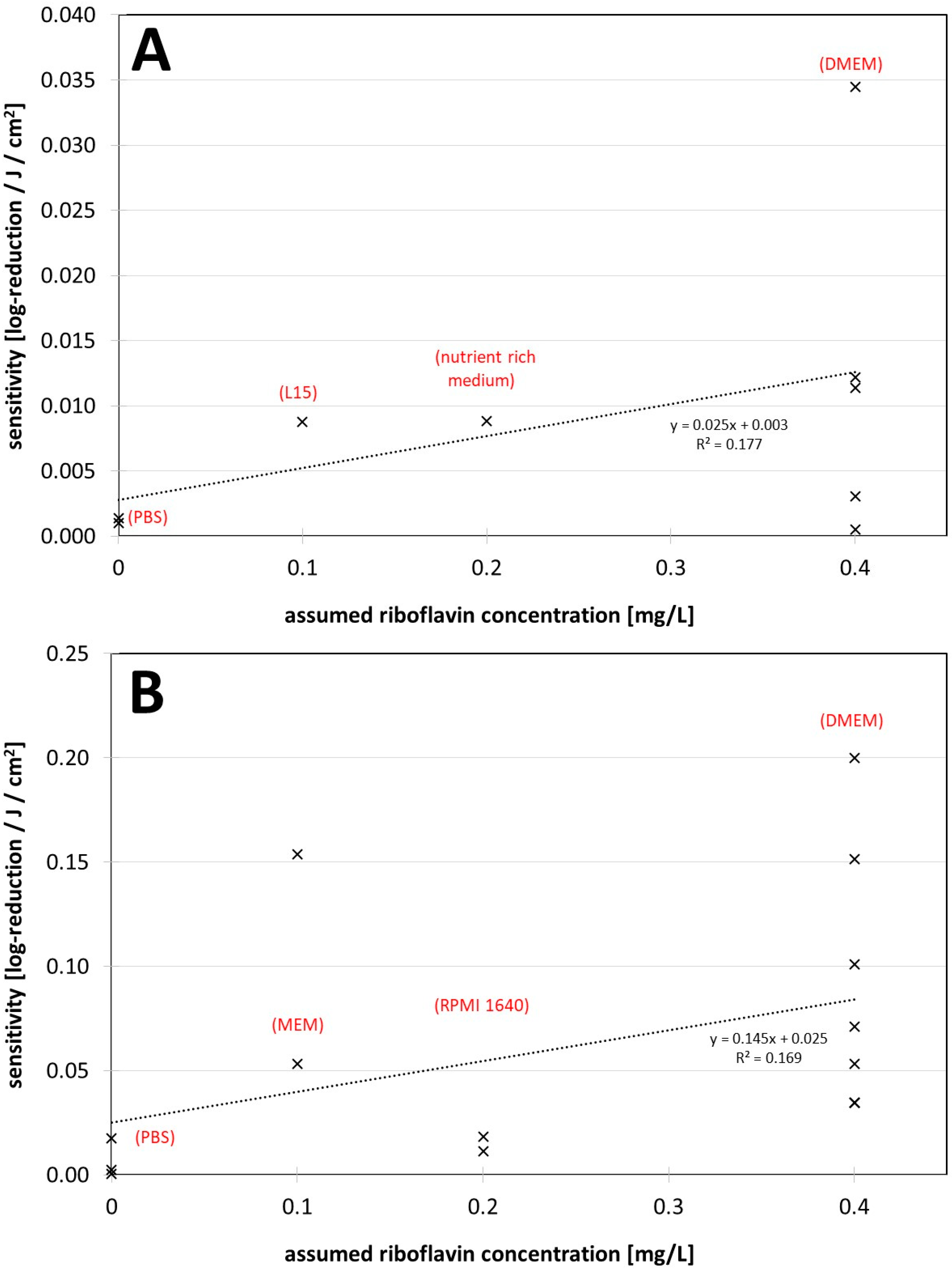
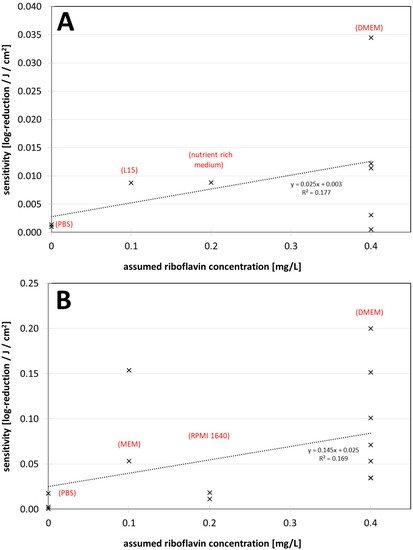 Figure 2. (A) Visible light sensitivity (inverse of the log-reduction dose) for viruses in different assumed riboflavin concentrations (media) for the non-enveloped viruses. (B) Visible light sensitivity for viruses in different assumed riboflavin concentrations (media) for enveloped viruses including coronaviruses. The dotted lines were derived by linear regression and are meant to visualize the dependence between riboflavin concentration (medium) and observed virus sensitivity.
Figure 2. (A) Visible light sensitivity (inverse of the log-reduction dose) for viruses in different assumed riboflavin concentrations (media) for the non-enveloped viruses. (B) Visible light sensitivity for viruses in different assumed riboflavin concentrations (media) for enveloped viruses including coronaviruses. The dotted lines were derived by linear regression and are meant to visualize the dependence between riboflavin concentration (medium) and observed virus sensitivity.4. The Findings regarding Virus Inactivation by Visible Light
35. Conclusions
It appears that riboflavin, and also possibly other media components [25,39,63][24][33][40] may have a major impact on photoinactivation results. DMEM especially might lead to higher virus sensitivities and should be used with caution in such experiments. Authors of future studies should consider this. As long as it cannot be excluded that the medium has an influence on photoinactivation of (corona-) viruses, it should be mentioned in publications that virus reduction with visible light may be quite different under other conditions, such as in air. This is especially important in the ongoing corona pandemic, where frightened citizens seek protective measures and companies might offer deceptive security based on misunderstood studies.References
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251.
- Kampf, G.; Voss, A.; Scheithauer, S. Inactivation of coronaviruses by heat. J. Hosp. Infect. 2020, 105, 348–349.
- Kratzel, A.; Todt, D.; V’kovski, P.; Steiner, S.; Gultom, M.; Thao, T.T.N.; Ebert, N.; Holwerda, M.; Steinmann, J.; Niemeyer, D.; et al. Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg. Infect. Dis. 2020, 26, 1592–1595.
- Hessling, M.; Hönes, K.; Vatter, P.; Lingenfelder, C. Ultraviolet irradiation doses for coronavirus inactivation—Review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control 2020, 15, 8.
- Hessling, M.; Hoenes, K.; Lingenfelder, C. Selection of parameters for thermal coronavirus inactivation—A data-based recommendation. GMS Hyg. Infect. Control 2020, 15, 16.
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 2003, 35, 17–24.
- Guffey, J.S.; Wilborn, J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed. Laser Surg. 2006, 24, 684–688.
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiol. Lett. 2008, 285, 227–232.
- Feuerstein, O.; Ginsburg, I.; Dayan, E.; Veler, D.; Weiss, E.I. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem. Photobiol. 2005, 81, 1186–1189.
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568.
- Plavskii, V.Y.; Mikulich, A.V.; Tretyakova, A.I.; Leusenka, I.A.; Plavskaya, L.G.; Kazyuchits, O.A.; Dobysh, I.I.; Krasnenkova, T.P. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J. Photochem. Photobiol. B 2018, 183, 172–183.
- Cieplik, F.; Spath, A.; Leibl, C.; Gollmer, A.; Regensburger, J.; Tabenski, L.; Hiller, K.-A.; Maisch, T.; Schmalz, G. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin. Oral Investig. 2014, 18, 1763–1769.
- Hessling, M.; Spellerberg, B.; Hoenes, K. Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths—A review on existing data. FEMS Microbiol. Lett. 2016, 364, fnw270.
- Tomb, R.M.; White, T.A.; Coia, J.E.; Anderson, J.G.; MacGregor, S.J.; Maclean, M. Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380-480 nm Violet-Blue Light. Photochem. Photobiol. 2018, 94, 445–458.
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; van Erp, P.E.J.; van de Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and histological effects of blue light on normal skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21.
- McDonald, R.S.; Gupta, S.; Maclean, M.; Ramakrishnan, P.; Anderson, J.G.; Macgregor, S.J.; Meek, R.M.D.; Grant, M.H. 405 nm Light exposure of osteoblasts and inactivation of bacterial isolates from arthroplasty patients: Potential for new disinfection applications? Eur. Cell. Mater. 2013, 25, 204–214.
- Wang, T.; Dong, J.; Yin, H.; Zhang, G. Blue light therapy to treat candida vaginitis with comparisons of three wavelengths: An in vitro study. Lasers Med. Sci. 2020, 35, 1329–1339.
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Investig. Dermatol. 2010, 130, 259–269.
- Bumah, V.V.; Masson-Meyers, D.S.; Awosika, O.; Zacharias, S.; Enwemeka, C.S. The viability of human cells irradiated with 470-nm light at various radiant energies in vitro. Lasers Med. Sci. 2021, 36, 1661–1670.
- Makdoumi, K.; Hedin, M.; Bäckman, A. Different photodynamic effects of blue light with and without riboflavin on methicillin-resistant Staphylococcus aureus (MRSA) and human keratinocytes in vitro. Lasers Med. Sci. 2019, 34, 1799–1805.
- Ramakrishnan, P.; Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Grant, M.H. Differential sensitivity of osteoblasts and bacterial pathogens to 405-nm light highlighting potential for decontamination applications in orthopedic surgery. J. Biomed. Opt. 2014, 19, 105001.
- Dai, T.; Gupta, A.; Huang, Y.-Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: Efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245.
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 2014, 209, 1963–1971.
- Grzelak, A.; Rychlik, B.; Bartosz, G. Light-dependent generation of reactive oxygen species in cell culture media. Free Radic. Biol. Med. 2001, 30, 1418–1425.
- Ruane, P.H.; Edrich, R.; Gampp, D.; Keil, S.D.; Leonard, R.L.; Goodrich, R.P. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion 2004, 44, 877–885.
- Marschner, S.; Goodrich, R. Pathogen Reduction Technology Treatment of Platelets, Plasma and Whole Blood Using Riboflavin and UV Light. Transfus. Med. Hemother. 2011, 38, 8–18.
- Faddy, H.M.; Prow, N.A.; Fryk, J.J.; Hall, R.A.; Keil, S.D.; Goodrich, R.P.; Marks, D.C. The effect of riboflavin and ultraviolet light on the infectivity of arboviruses. Transfusion 2015, 55, 824–831.
- Faddy, H.M.; Fryk, J.J.; Watterson, D.; Young, P.R.; Modhiran, N.; Muller, D.A.; Keil, S.D.; Goodrich, R.P.; Marks, D.C. Riboflavin and ultraviolet light: Impact on dengue virus infectivity. Vox Sang. 2016, 111, 235–241.
- Elikaei, A.; Hosseini, S.M.; Sharifi, Z. Inactivation of model viruses and bacteria in human fresh frozen plasma using riboflavin and long wave ultraviolet rays. Iran. J. Microbiol. 2017, 9, 50–54.
- Callahan, S.M.; Wonganan, P.; Obenauer-Kutner, L.J.; Sutjipto, S.; Dekker, J.D.; Croyle, M.A. Controlled inactivation of recombinant viruses with vitamin B2. J. Virol. Methods 2008, 148, 132–145.
- Keil, S.D.; Ragan, I.; Yonemura, S.; Hartson, L.; Dart, N.K.; Bowen, R. Inactivation of severe acute respiratory syndrome coronavirus 2 in plasma and platelet products using a riboflavin and ultraviolet light-based photochemical treatment. Vox Sang. 2020, 115, 495–501.
- Zhou, Z.-Y.; Bi, X.-X. Experimental studies on the inactivation of HBV in blood via riboflavin photochemical treatment. Exp. Ther. Med. 2017, 13, 222–224.
- Tomb, R.M.; Maclean, M.; Coia, J.E.; Graham, E.; McDonald, M.; Atreya, C.D.; MacGregor, S.J.; Anderson, J.G. New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light Against Feline Calicivirus as a Model for Norovirus Decontamination. Food Environ. Virol. 2016, 9, 159–167.
- Biasin, M.; Strizzi, S.; Bianco, A.; Macchi, A.; Utyro, O.; Pareschi, G.; Loffreda, A.; Cavalleri, A.; Lualdi, M.; Trabattoni, D.; et al. UV-A and UV-B Can Neutralize SARS-CoV-2 Infectivity. medRxiv 2021.
- Rathnasinghe, R.; Jangra, S.; Miorin, L.; Schotsaert, M.; Yahnke, C.; Garcίa-Sastre, A. The virucidal effects of 405 nm visible light on SARS-CoV-2 and influenza A virus. Sci. Rep. 2021, 11, 19470.
- Tsugita, A.; Okada, Y.; Uehara, K. Photosensitized inactivation of ribonucleic acids in the presence of riboflavin. Biochim. Biophys. Acta—Nucleic Acids Protein Synth. 1965, 103, 360–363.
- Appleyard, G. The photosensitivity of Semliki Forest and other viruses. J. Gen. Virol. 1967, 1, 143–152.
- Cutchins, E.C.; Dayhuff, T.R. Photoinactivation of measles virus. Virology 1962, 17, 420–425.
- Wallis, C.; Trulock, S.; Melnick, J.L. Inherent photosensitivity of herpes virus and other enveloped viruses. J. Gen. Virol. 1969, 5, 53–61.
- Nemo, G.J.; Cutchins, E.C. Effect of visible light on canine distemper virus. J. Bacteriol. 1966, 91, 798–802.
- Tomb, R.M.; Maclean, M.; Herron, P.R.; Hoskisson, P.A.; MacGregor, S.J.; Anderson, J.G. Inactivation of Streptomyces phage C31 by 405 nm light: Requirement for exogenous photosensitizers? Bacteriophage 2014, 4, e32129.
- Kingsley, D.; Kuis, R.; Perez, R.; Basaldua, I.; Burkins, P.; Marcano, A.; Johnson, A. Oxygen-dependent laser inactivation of murine norovirus using visible light lasers. Virol. J. 2018, 15, 117.
- Kingsley, D.H.; Perez-Perez, R.E.; Boyd, G.; Sites, J.; Niemira, B.A. Evaluation of 405-nm monochromatic light for inactivation of Tulane virus on blueberry surfaces. J. Appl. Microbiol. 2018, 124, 1017–1022.
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074.
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612.
- Booth, J.C.; Stern, H. Photodynamic inactivation of rubella virus. J. Med. Microbiol. 1972, 5, 515–528.
- Wallis, C.; Melnick, J.L. Irreversible photosensitization of viruses. Virology 1964, 23, 520–527.
- Vatter, P.; Hoenes, K.; Hessling, M. Blue light inactivation of the enveloped RNA virus Phi6. BMC Res. Notes 2021, 14, 187.
