Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 6 by Jason Zhu and Version 8 by Lindsay Dong.
In recent years, scholars around the world have worked on improving recycle aggregates in concrete and broadening the scope of applications of recycled concrete. This paper reviews the findings of research on the effects of recycled fine aggregates (RFAs) on the permeability, drying shrinkage, carbonation, chloride ion penetration, acid resistance, and freeze–thaw resistance of concrete has been reviewed. The results show that the content of old mortar and the quality of recycled concrete are closely related to the durability of prepared RFA concrete.
- Freeze
- Drying Shrinkage
- Carbonation
- recycled fine aggregate concrete
1. Impermeability
Impermeability is a characteristic of the durability of concrete and is closely related to other durability characteristics. For example, water-soluble chloride ions, CO2, and sulfate can enter concrete, or other external factors can cause damage to the concrete, making the service life of concrete greatly affected and thus becoming a global concern [1][2][3]. The impermeability of concrete is mainly determined by its own microscopic properties such as pore size and interfacial transition zone. The impermeability of RFAc is mainly influenced by the replacement rate of RFA, W/C, and mineral admixtures. It was found that the addition of admixtures and additives to concrete can improve the microscopic effect and achieve improved impermeability [4][5][6].
The poor grading of RFA particles with a large number of voids on the surface and micro cracks attached to the old mortar renders the prepared RCAc less dense, so the level of RCAc impermeability decreases with the increase in the RCA substitution rate [7][8]. In self-compacting concrete (SSC), water absorption increases as the RFA substitution rate increases, which is caused by the high porosity and high water absorption of RFA [9][10]. In a sulfate environment, RFAc exhibited better permeability resistance than normal concrete. The water absorption of RFAc exposed to a sulfate environment decreased by an average of 25% compared to normal concrete [11].
The water–cement ratio is an important parameter in the preparation of concrete, which will affect the permeability resistance of concrete. A water–cement ratio of 0.65 has a lower impermeability rating than RFAc with a water–cement ratio of 0.55, indicating that the higher the water–cement ratio, the worse the impermeability of RFAc, because the higher the water–cement ratio, the slower the hydration of the RFAc cement and the more pores it produces, which leads to the poor compactness of RFAc [7][8][12].
Due to its volcanic ash nature, fly ash (FA) has been widely used as a replacement for silicate cement in engineering construction [13]. The addition of fine fly ash to concrete can promote hydration reactions, zeolite reactions, accumulation effects, and nucleation effects [14]. Thus, FA can participate in the early hydration reaction and increase the hydration products to fill the pores and increase the density of the concrete [15]. In self-compacting concrete mixed with 100% RFA (SSC) and 10% (FA), the permeability of SSC prepared with different water–cement ratios was found to be significantly lower compared to the control group without FA [12]. The physical and chemical properties of RFA are defective, which weakens the transition zone at the interface of the prepared RAC. In order to improve the ITZ of RAC, several researchers have developed different concrete mixing methods. Figure 1 shows the double mixing method (DM) [16] and the triple mixing method (TM) [17] as well as the optimized triple mixing method (OTM). The difference between the OTM and the other two methods is that the water-reducing agent (SP) is added in a different order. In the OTM, SP is added together with other gelling materials to promote additional gelling. The dispersion of this material further increases the content of additional gelling material in the ITZ, which can better exploit the zeolite effect at a later stage, such that the use of the OTM can result in a permeability rating of 8 for RAC [18].
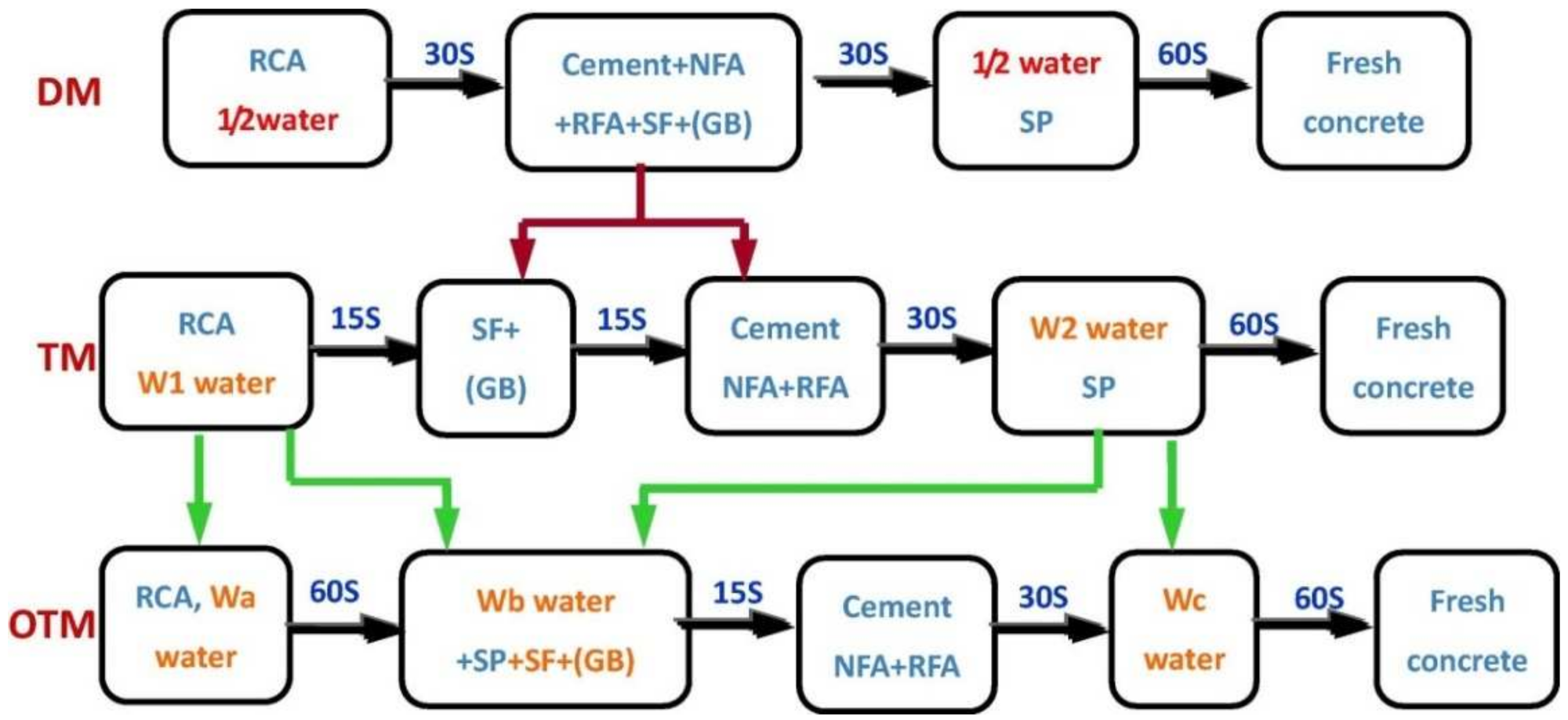
Figure 1. Comparison of mixing methods for the preparation of RAC [18] (Wa equals 60–80% of the product of the weight of RA multiplied by its water absorption, Wb equals the weight percentage of residual water of SCM in the total gelling material, and Wc = mixed water − Wb − Wa).
The water absorption rate of RFA is much higher than that of natural fine aggregate. RFA absorbs water when freshly mixed with concrete, which has an effect on the mechanical properties and durability of concrete, so the water content of RFA before mixing should not be neglected. Therefore, the effect of RFA in three states of air-drying (AD), oven-drying (OD), and saturated surface drying (SSD) on the permeability of the prepared RFAc was investigated, and it was found that the permeability of these states at the same RFA replacement rate was ordered as follows: SSD > AD > OD. This finding was different from that of RCA. The permeability resistance of concrete prepared from RCA with different moisture contents was ordered as follows: SSD < AD < OD [19]. The reason for this may be that the particle size of RFA is smaller than RCA, but the specific surface area is larger, so the water can become more confined [20].
The addition of RFA has a serious effect on the permeability of concrete. An increase in the RFA replacement rate and the W/C ratio will lead to a decrease in the permeability resistance. However, the use of saturated surface-dried RFA, optimized mixing methods, and the addition of fly ash can improve the impermeability of RFAc. In addition to fly ash, silica fume, metakaolin, and other mineral admixtures also have zeolite properties, so the impermeability of RFAc mixed with silica fume and higher mineral admixtures is also beneficial.
2. Drying Shrinkage
Drying shrinkage requirements are already an important part of project specifications [21]. The evaporation of water from the interior of concrete in a dry environment results in a change in concrete dimensions with time. The drying shrinkage process starts with the evaporation of free water from the pores, followed by the evaporation of adsorbed water from the very small capillary pores; the evaporation of free water is not related to drying shrinkage, but the evaporation of adsorbed water leads to the shrinkage and internal generation of tensile stresses to the extent that drying shrinkage cracks appear in the concrete [22]. As for the coarse aggregate concrete, it was found that the “electric arc furnace slag” improves the strength and modulus of elasticity and leads to shrinkage reduction of concrete [23][24].
The drying shrinkage value increases as the RFA substitution rate increases, and when the substitution rate is 100%, the drying shrinkage value of RFAc is twice as high as that of normal concrete [25]. The drying shrinkage value of RFAc increases with the substitution rate and decreases as the interim period increases. One study found that the drying shrinkage value of RFAc with a 30% substitution rate was 14.0% lower than that of normal concrete and 8.2% higher than that of normal concrete with a 100% substitution rate at 90 days of the prophase [26] (Figure 2). It has also been found that the relationship between drying shrinkage values and the RFA substitution rate is divided by 30%; drying shrinkage values after a substitution rate of 30% increase as the substitution rate increases, and the substitution rate before a substitution rate of 30% has no significant effect on drying shrinkage values [27].
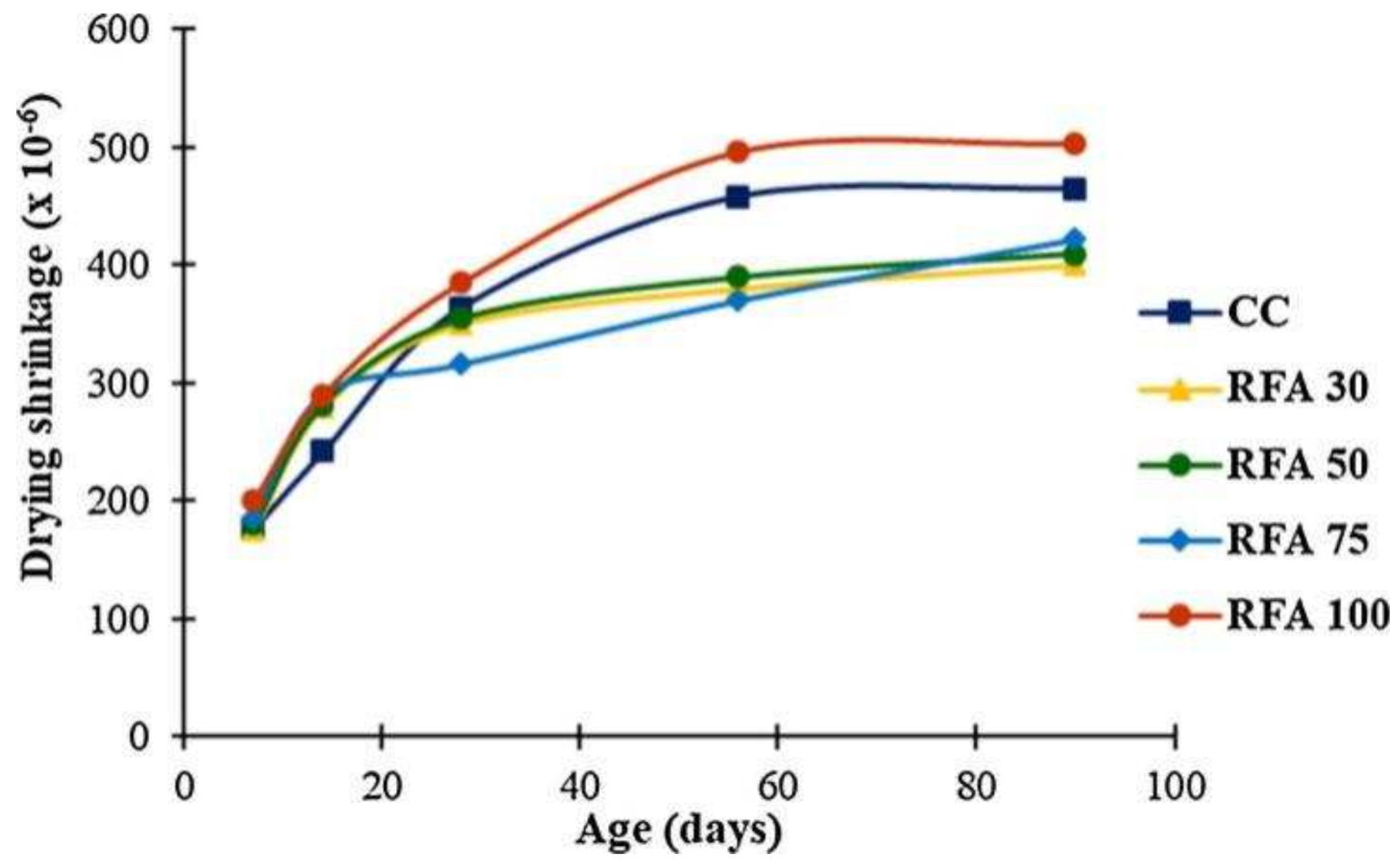
Figure 2. Dry shrinkage of RS (river sand) and RFA concrete [26].
Different sources of RFA also have an important effect on RFAc, and the study of RFAc prepared from different sources of RFA has great significance for the application of waste concrete in practical engineering. RFA from recycled laboratory-produced concrete and RFA from recycled precast concrete, both of which have similar strengths, were studied for drying shrinkage values of RFAc under both recycling routes and were found to be similar [28]. However, in another study, which also investigated RFA from two different sources, RFA1 from flat-plate concrete and RFA2 from composite beam specimen concrete, drying shrinkage tests on RFAc prepared from both RFAs at 0%, 50, and 100% substitution rates were conducted, and it was found that, regardless of the substitution rate of RFA, the drying shrinkage values of RFAc prepared from RFA2 were lower than that of RFAc prepared by RFA1. The differences in drying shrinkage values exhibited by RFAc prepared by the two sources are mainly related to the physical properties of RFA: (1) the density of RFA2 is greater than that of RFA1 (RFA1: 2222 kg/m3, RFA2: 2326 kg/m3), indicating that RFA1 contains a higher amount of old cement paste than RFA2; and (2) the water absorption is lower than that of RFA1 (RFA1: 11.2%, RFA2: 8.1%). These two differences in physical properties indicate that the water content of RFAc prepared by RFA1 is higher than that of RFAc prepared by RFA2 and that the high water content has a low binding effect on concrete [29] (Figure 3).
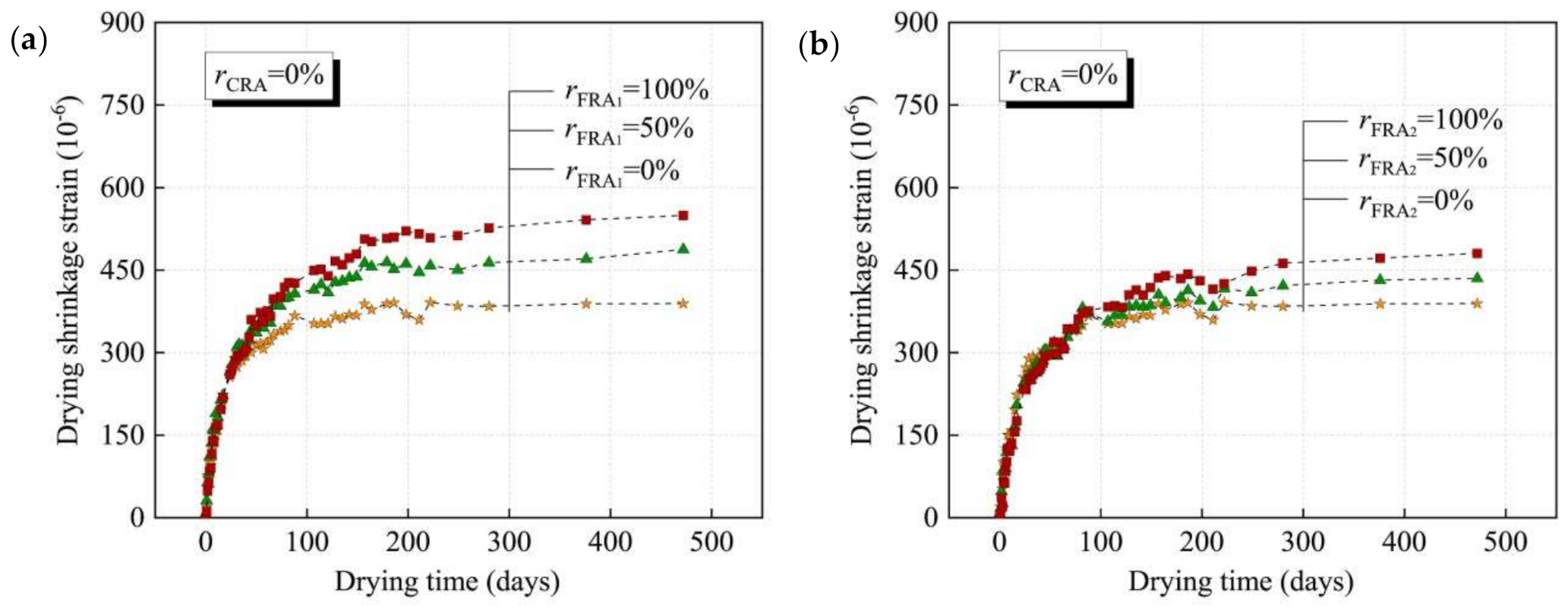
Figure 3. Development of dry shrinkage of concrete with time using different sources of FRA [29]: (a) concrete with FRA1, rCRA = 0%; (b) concrete with FRA2, rCRA = 0%.
The drying shrinkage of RFAc with a 20% RFA replacement rate and an effective water–cement ratio of 0.43 was similar to that of the control with a water–cement ratio of 0.45 at the same amount of RFAc mixing water, but the drying shrinkage of RFAc with a 30% RFA replacement rate and an effective water–cement ratio of 0.41 was lower than that of the control [30]. SP is a very frequently used admixture, and its addition reduces the amount of mixed water in the workable fresh concrete mixture, reducing the water–cement ratio for the same amount of cement and thus improving the performance of RFA [31][32][33]. The effect of different SPs on the drying shrinkage of RFAc prepared with different replacement rates of RFA was investigated, mainly comparing RFAc without SP, with conventional plasticizer (SP1), and with high-performance and high-efficiency plasticizer (SP2), and it was found that the drying shrinkage values of RFAc without SP increased to 28 and 57% at 7 and 91 days, respectively; the drying shrinkage values of RFAc with SP1 increased at 7 days to 44%, while the drying shrinkage value at 91 days decreased to 2%; the drying shrinkage value of RFAc7d with SP2 increased to 38%, while the drying shrinkage value at 91 days decreased to 30%, which shows that the addition of SP was effective in reducing the drying shrinkage value of RFAc, and the effectiveness of SP2 was higher than that of SP1 [34].
Silica fume (SF), produced during the smelting process of the ferrosilicon industry, has a silica content of up to 75% and is an effective volcanic ash material [35]. Due to the large amount of water absorption of the old mortar attached to the RA surface, it can lead to an increase in the drying shrinkage value of SSC prepared with RFA. It was found that the drying shrinkage value of the ternary RFA SSC prepared with finely ground blast furnace slag (GGBFS) and SF instead of cement was greatly reduced, on the one hand because the two external admixtures promoted hydration and on the other hand because the ultrafine material filled the pores inside the concrete [12].
Different mixing methods also have an effect on the performance of concrete. RFA is one of the important reasons for the performance of concrete due to its excessive water absorption; however, the use of moisture in the two-part addition, i.e., the two-stage mixing method (TSMA), can effectively prevent the excessive water absorption of RFA [36]. The performance of concrete with different RFA replacement rates prepared by the normal mixing method and TSMA was investigated, and it was found that the drying shrinkage values of all RFAc using TSMA were lower than those of the concrete prepared by the normal mixing method, mainly because of the higher compactness and better ITZ of RFAc prepared by TSMA [37].
In summary, the drying shrinkage values of the concrete prepared by the RFA substitution of sand became larger with the increase of the RFA substitution rate. In order to reduce the effect of RFA on the drying shrinkage value of concrete, the drying shrinkage value can be effectively reduced by adding admixture (SP) and mineral admixture, and the drying shrinkage value of RFAc with TSMA is also smaller than that of RFAc with a normal mixture. Drying shrinkage is a long-term performance feature of concrete, which is of great significance to concrete in the process of use, but RFAc is significantly affected by RFA, so it is necessary to consider an optimal replacement rate of RFA when preparing RFAc. Therefore, an optimal replacement rate of RFA needs to be considered when preparing RAFc, and the effect of RFA on the drying shrinkage of concrete should be reduced by other means.
3. Carbonation Resistance
The amount of CO2 in the air is about 0.03%. The pores present in concrete allow CO2 to diffuse within it and react with hydration products to form a carbonation process. Carbonation is mainly a chemical reaction between carbon dioxide (CO2) and calcium hydroxide (CH), hydrated calcium silicate (C-S-H), or bentonite (AFt) [38]. Determining the carbonation resistance of RFAc is fundamental to the study of its durability, since concrete is exposed to natural environments that induce carbonation corrosion for long periods of time, and the rate of carbonation is directly related to the lifetime of concrete. The carbonation resistance of RFAc is mainly related to the minimum particle size of RFA, the substitution rate, the W/C ratio, and the mineral external admixtures.
The smaller the RFA particle size, the higher the amount of old mortar adhered to the surface, so the carbonation depth of RFAc increases instead as the minimum particle size of the RFA decreases [39]. CO2 curing is one of the means of treating RFA, and CO2 curing can fill the pores and cracks on the surface of the aggregate and improve the performance of the old and new ITZ [40]. Carbonation studies were conducted on concrete prepared from CO2-cured recycled coarse and fine aggregates; it was found that the treated RFAc had improved carbonation resistance compared to the untreated one, with the maximum carbonation resistance depth of the former being about 42.5% lower than that of the latter (Figure 4), and it also showed the same trend under freeze–thaw cycling conditions [41].
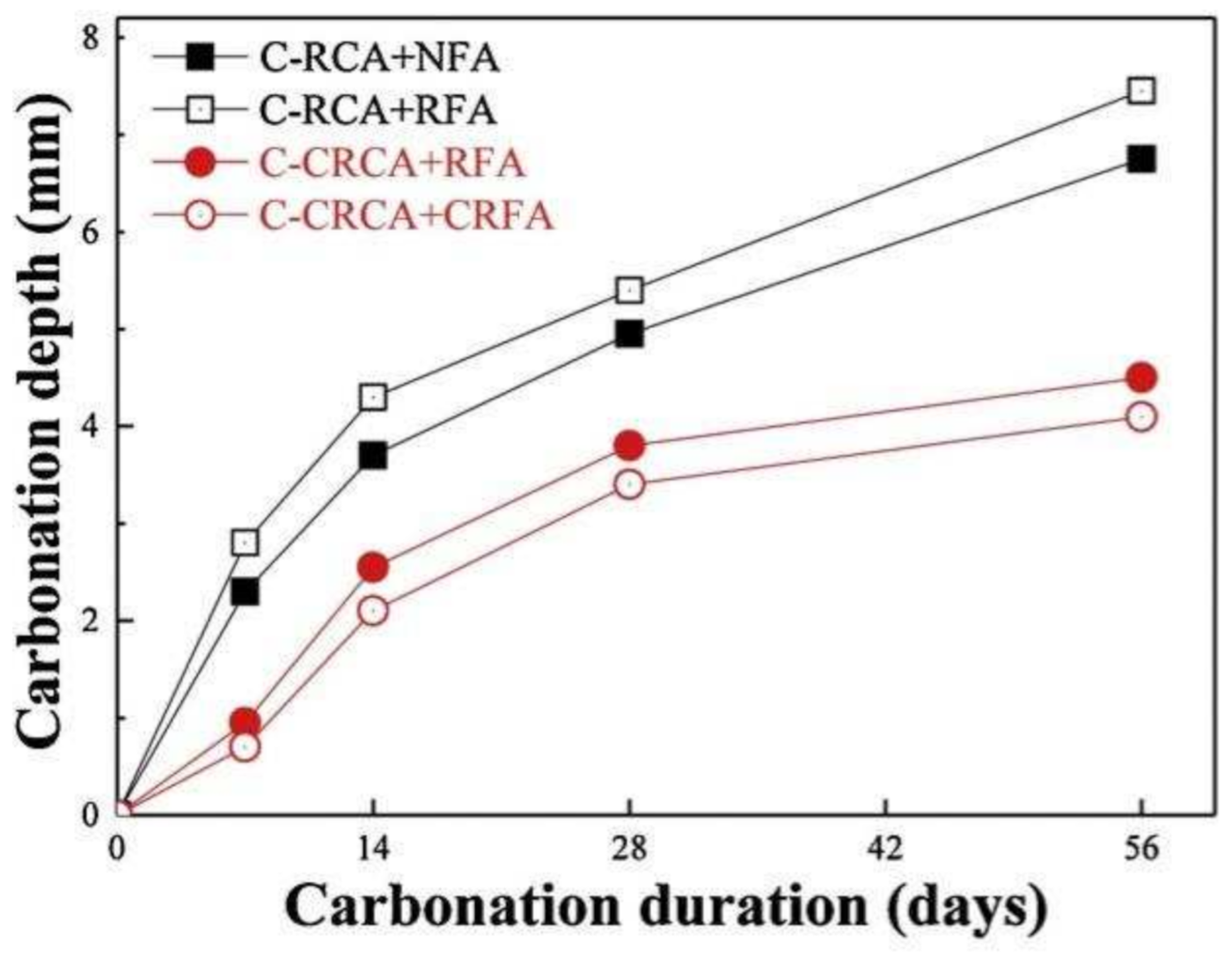
Figure 4. Carbonation behavior of CRA concrete exposed to carbonation [41].
When the replacement rate of RFA in concrete is 30 and 100%, the carbonation depth of concrete CO2 increases by about 40 and 110%, respectively, indicating that the resistance of RFAc to carbonation decreases as the replacement rate of RFA increases, which is caused by the internal capillarity of RFAc [39][42][43]. In self-compacting concrete (SSC), the depth of carbonation also increases with the increase in the substitution rate [44].
The lower carbonation resistance of RFAc compared to the control is not only due to the high porosity of RFA but also related to the increase in the effective W/C, which improves the diffusion of CO2 [45]. Fly ash is beneficial for the anticarbonation properties of RFAc; when fly ash replaced cement in the range of 10–30%, a replacement rate of 20% was found to be most beneficial for the anticarbonation properties of RFAc [39]. Some studies, however, have found that the carbonation depth of RFAc increased with increasing fly ash when the RFA substitution rate was below 30% [46]. In self-compacting concrete (SSC) incorporating RFA, it was found that partial age soils (MK) were beneficial to the anticarbonation properties, and SSC with a 50% RFA substitution rate and incorporating MK showed a 20 and 18% decrease in carbonation depth after 4 and 12 weeks of accelerated carbonation exposure, respectively, while SSC with a 100% RFA substitution rate and incorporating MK showed the same carbonation depth after 4 and 12 weeks of accelerated carbonation exposure as the control group. The reason for this may be the high reactivity of the added MK and volcanic ash products such as calcium hydroxide, which caused a large consumption of early hydrated calcium (CH) and thereby reduced the carbonation depth [44].
The carbonation depth increased with time, and the RFAc with SP2 (SikaPlast898) addition had a lower carbonation depth than both without plasticizer and with SP1 (Sikament 400 plus) RFAc at each prophase, mainly because the addition of SP2 decreased the effective w/b of RFAc. Thus, the internal porosity was lower, and the gas permeability was lower [47]. The resistance to carbonation of recycled concrete prepared by one generation of RFA (RAC1) and recycled concrete prepared by two generations of RFA (RAC2) was investigated at four bending stress levels (40, 70, 100, and 120%), and it was found that the carbonation depth increased with stress, except for the 70% stress level, and the carbonation depth of RAC2 was higher than that of RAC1 [43].
The particle size of RFA affects the carbonation resistance of RFAc, and the increase of the RFA substitution rate and W/C can negatively affect the carbonation resistance of RFAc. The anticarbonation performance of RFAc can be improved by the CO2 curing of RFA and the addition of mineral ex-dopant. CO2 curing is one of the methods that can be used to improve the quality of RFA, and mineral ex-dopant coating and microbial carbonate precipitation treatment can also improve the quality of RFA and thus the anticarbonation performance of RFAc.
4. Acid Resistance
Industrial production causes a large amount of sulfur dioxide emissions, as well as an increasing number of nitrogen oxides from vehicle exhaust, which are the main components that contribute to the production of acid rain (NOx) and sulfur dioxide (SO2) [48]. The concrete structure of buildings exposed to acid rain for long periods of time is affected by the deterioration of concrete and the rusting of reinforcements. The deterioration of concrete by acid is the reaction of sulfuric acid with calcium hydroxide to produce gypsum, as shown in Equations, and gypsum formation expands concrete and accelerates corrosion. RFAc containing 100% RFA is less durable than natural sand concrete because of such properties as the internal capillarity of RFAc [49].
Ca(OH)2 + H2SO4 → CaSO4 · 2H2O
CaO · Al2O3 · 12H2O + 3(CaSO4 · 2H2O) + 14H2O → CaO · Al2O3 · 3CaSO4 · 32H2O
CaO · SiO2 · 2H2O + H2SO4 → CaSO4 + Si(OH)4 + H2O
The acid resistance of concrete depends mainly on its percentage reduction in mass and compressive strength in acid solutions. The reduction in mass loss and compressive strength in a 3% sulfuric acid solution was discussed by adding RFA with different substitution rates to the activated concrete, which was considered from two sources, one from normal concrete and the other from activated powder concrete. It was found that the mass loss and compressive strength of the activated powder concrete prepared from both sources of RFA subjected to sulfuric acid attack increased as the substitution rate of RFA increased, but both had good acid resistance [50]. This is mainly due to the reaction of sulfuric acid with calcium hydroxide through the porous activated powder concrete to produce gypsum, which expands calcium salts in the pores in aqueous solutions and finally leads to a decrease in the mass and compressive strength of the activated powder concrete [51]. The acid resistance of high-performance concrete (HPC) in sulfate solutions and sulfuric acid solutions was studied by adding 20% RCA and RFA, and it was found that HPC had good acid resistance in sulfate solutions, probably due to the addition of SF, but a severe loss of strength in sulfuric acid solutions [52]. In concentrated hydrochloric acid and concentrated sulfuric acid, the corresponding mass loss of plain concrete with added RFA increased with the soaking time and the substitution rate, and the mass loss of RFAc with a 30% substitution rate after 90 days was lower than that of the control, while the mass loss of RFAc with a 100% substitution rate was higher than that of the control. This was because the RFA with a 100% substitution rate was finer and contained more pores and old cement mortar [26]. Similar findings were found in another study on the effect of RFAs on the acid resistance of alkali-activated slag concrete (AASC), a new environmentally friendly cementitious material, where AASC with 0, 50, and 100% RFA replacement rates were exposed to a 3% sulfuric acid solution for 180 days, and it was found that the quality of AASC decreased as the replacement rate increased, but even after 180 days of exposure, the maximum mass loss of AASC was less than 20% (as shown in the figure), which was mainly due to the presence of GGBS in AASC [53] (Figure 5). The acid resistance of SSC prepared with coarse and fine recycled aggregates in 5% sulfate was better than the control. Meanwhile, the effect of natural volcanic ash (PZ) on the recycled SSC was investigated, and it was found that the addition of PZ improved the acid resistance of the recycled SSC, with 20% PZ dosing being optimal [54].
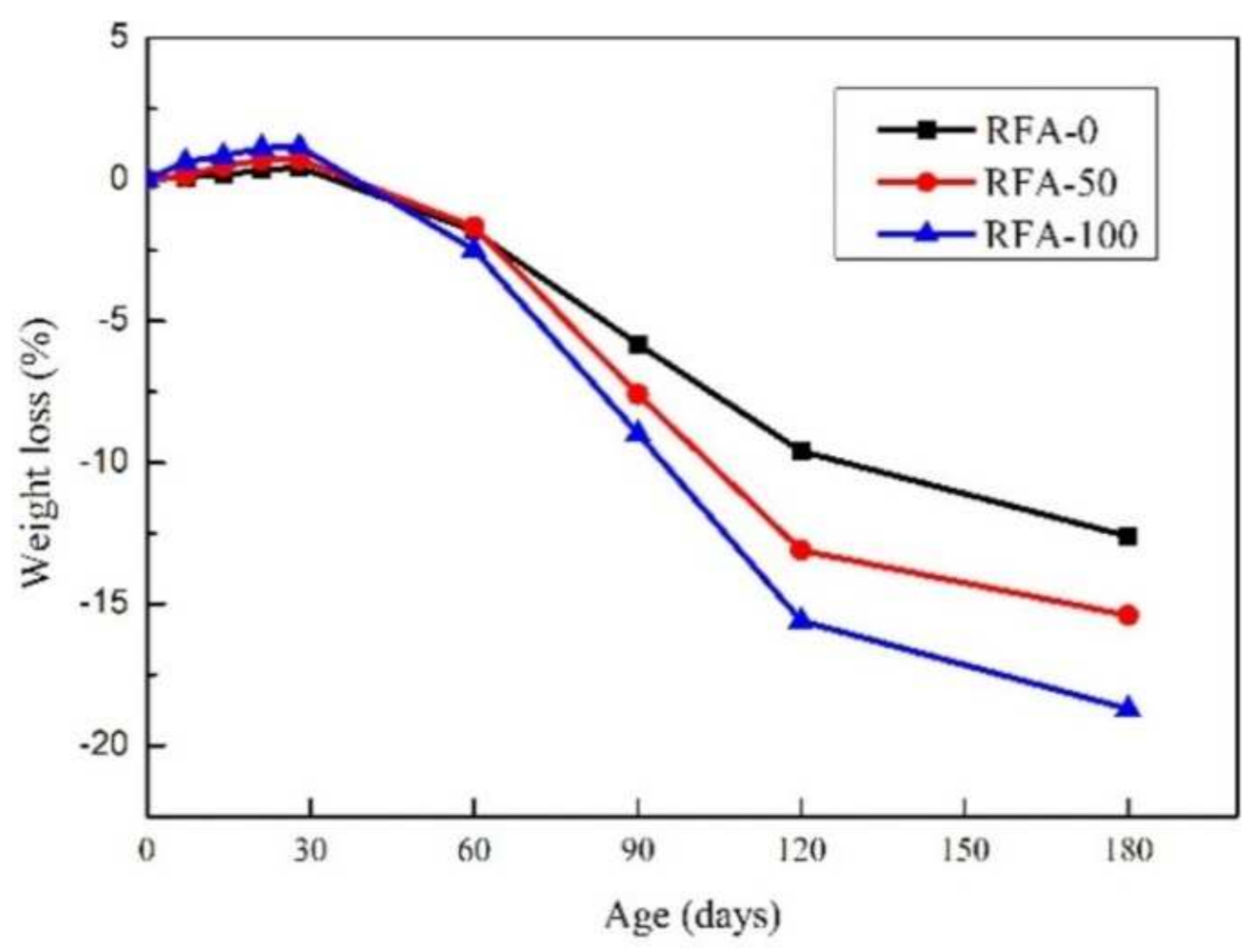
Figure 5. Weight loss of alkali−activated slag concrete [53].
Different types of concrete supplemented with RFA showed mass loss and compressive strength reduction in acid solutions. The more significant the mass loss and compressive strength reduction was, the more the replacement rate of RFA increased. The incorporation of natural volcanic ash into SSC incorporated with RFA improves the acid resistance. An appropriate RFA substitution rate should be considered for RFAc in acidic environments, and a certain amount of natural volcanic ash can be considered to improve the acid resistance.
References
- Barbucci, A.; Delucchi, M.; Cerisola, G. Organic coatings for concrete protection: Liquid water and water vapour permeabilities. Prog. Org. Coat. 1997, 30, 293–297.
- Wang, Y.; Chen, G.; Wan, B.; Lin, H.; Zhang, J. Behavior of innovative circular ice filled steel tubular stub columns under axial compression. Constr. Build. Mater. 2018, 171, 680–689.
- Brenna, A.; Bolzoni, F.; Beretta, S.; Ormellese, M. Long-term chloride-induced corrosion monitoring of reinforced concrete coated with commercial polymer-modified mortar and polymeric coatings. Constr. Build. Mater. 2013, 48, 734–744.
- Zhang, Y.; Kong, X. Influences of superplasticizer, polymer latexes and asphalt emulsions on the pore structure and impermeability of hardened cementitious materials. Constr. Build. Mater. 2014, 53, 392–402.
- Güneyisi, E.; Gesoğlu, M.; Booya, E.; Mermerdaş, K. Strength and permeability properties of self-compacting concrete with cold bonded fly ash lightweight aggregate. Constr. Build. Mater. 2015, 74, 17–24.
- Duan, P.; Shui, Z.; Chen, W.; Shen, C. Efficiency of mineral admixtures in concrete: Microstructure, compressive strength and stability of hydrate phases. Appl. Clay Sci. 2013, 83, 115–121.
- Sun, Y.; Li, D.; Xu, Y.; Huang, H. Experimental study on mechanical properties and durability of recycled fine aggregate concrete in actual production. Build. Struct. 2019, 49, 81–84.
- Mardani-Aghabaglou, A.; Tuyan, M.; Ramyar, K. Mechanical and durability performance of concrete incorporating fine recycled concrete and glass aggregates. Mater. Struct. 2015, 48, 2629–2640.
- Sasanipour, H.; Aslani, F. Durability properties evaluation of self-compacting concrete prepared with waste fine and coarse recycled concrete aggregates. Constr. Build. Mater. 2020, 236, 117540.
- Kapoor, K.; Singh, S.; Singh, B. Permeability of self-compacting concrete made with recycled concrete aggregates and metakaolin. J. Sustain. Cem.-Based Mater. 2017, 6, 293–313.
- Mostofinejad, D.; Hosseini, S.M.; Nosouhian, F.; Ozbakkaloglu, T.; Tehrani, B.N. Durability of concrete containing recycled concrete coarse and fine aggregates and milled waste glass in magnesium sulfate environment. J. Build. Eng. 2020, 29, 101182.
- Gesoglu, M.; Güneyisi, E.; Öz, H.Ö.; Yasemin, M.T.; Taha3, I. Durability and shrinkage characteristics of self-compacting concretes containing recycled coarse and/or fine aggregates. Adv. Mater. Sci. Eng. 2015, 2015, 278296.
- Hemalatha, T.; Ramaswamy, A. A review on fly ash characteristics–Towards promoting high volume utilization in developing sustainable concrete. J. Clean. Prod. 2017, 147, 546–559.
- Chindaprasirt, P.; Jaturapitakkul, C.; Sinsiri, T. Effect of fly ash fineness on microstructure of blended cement paste. Constr. Build. Mater. 2007, 21, 1534–1541.
- Zhang, M.-H.; Li, L.; Paramasivam, P. Shrinkage of high-strength lightweight aggregate concrete exposed to dry environment. Mater. J. 2005, 102, 86–92.
- Tam, V.W. Comparing the implementation of concrete recycling in the Australian and Japanese construction industries. J. Clean. Prod. 2009, 17, 688–702.
- Kong, D.; Lei, T.; Zheng, J.; Ma, C.; Jiang, J.; Jiang, J. Effect and mechanism of surface-coating pozzalanics materials around aggregate on properties and ITZ microstructure of recycled aggregate concrete. Constr. Build. Mater. 2010, 24, 701–708.
- Zhang, W.; Wang, S.; Zhao, P.; Lu, L.; Cheng, X. Effect of the optimized triple mixing method on the ITZ microstructure and performance of recycled aggregate concrete. Constr. Build. Mater. 2019, 203, 601–607.
- Mefteh, H.; Kebaïli, O.; Oucief, H.; Berredjem, L.; Arabi, N. Influence of moisture conditioning of recycled aggregates on the properties of fresh and hardened concrete. J. Clean. Prod. 2013, 54, 282–288.
- Zhuang, Y.; Liang, Y.; Nabizadeh, A.; Ng, Z.; Ji, T. Influence of the moisture state of recycled fine aggregate on the impermeability of concrete. Mater. Test. 2019, 61, 991–998.
- Videla, C.; Aguilar, C. An updated look at drying shrinkage of Portland and blended Portland cement concretes. Mag. Concr. Res. 2006, 58, 459–476.
- Mokarem, D.W.; Weyers, R.E.; Lane, D.S. Development of a shrinkage performance specifications and prediction model analysis for supplemental cementitious material concrete mixtures. Cem. Concr. Res. 2005, 35, 918–925.
- De Domenico, D.; Faleschini, F.; Pellegrino, C.; Ricciardi, G. Structural behavior of RC beams containing EAF slag as recycled aggregate: Numerical versus experimental results. Constr. Build. Mater. 2018, 171, 321–337.
- Arribas, I.; Santamaria, A.; Ruiz, E.; Ortega-Lopez, V.; Manso, J.M. Electric arc furnace slag and its use in hydraulic concrete. Constr. Build. Mater. 2015, 90, 68–79.
- Jang, S.-J.; Yun, H.-D. Mechanical properties of ready-mixed concrete incorporating fine recycled aggregate. Mag. Concr. Res. 2015, 67, 621–632.
- Kirthika, S.; Singh, S. Durability studies on recycled fine aggregate concrete. Constr. Build. Mater. 2020, 250, 118850.
- Guo, Z.; Chen, C.; Lehman, D.E.; Xiao, W.; Zheng, S.; Fan, B. Mechanical and durability behaviours of concrete made with recycled coarse and fine aggregates. Eur. J. Environ. Civ. Eng. 2020, 24, 171–189.
- Pedro, D.D.; De Brito, J.; Evangelista, L. Structural concrete with simultaneous incorporation of fine and coarse recycled concrete aggregates: Mechanical, durability and long-term properties. Constr. Build. Mater. 2017, 154, 294–309.
- Zhang, H.; Wang, Y.; Lehman, D.E.; Geng, Y.; Kuder, K. Time-dependent drying shrinkage model for concrete with coarse and fine recycled aggregate. Cem. Concr. Compos. 2020, 105, 103426.
- Zega, C.J.; Di Maio, Á.A. Use of recycled fine aggregate in concretes with durable requirements. Waste Manag. 2011, 31, 2336–2340.
- Pereira, P.; Evangelista, L.; Brito, J.D. The effect of superplasticisers on the workability and compressive strength of concrete made with fine recycled concrete aggregates. Constr. Build. Mater. 2011, 28, 722–729.
- Pereira, P.; Evangelista, L.; De Brito, J. The effect of superplasticizers on the mechanical performance of concrete made with fine recycled concrete aggregates. Cem. Concr. Compos. 2012, 34, 1044–1052.
- Barbudo, A.; De Brito, J.; Evangelista, L.; Bravo, M.; Agrela, F. Influence of water-reducing admixtures on the mechanical performance of recycled concrete. J. Clean. Prod. 2013, 59, 93–98.
- Cartuxo, F.; De Brito, J.; Evangelista, L.; Jiménez, J.R.; Ledesma, E.F. Rheological behaviour of concrete made with fine recycled concrete aggregates—Influence of the superplasticizer. Constr. Build. Mater. 2015, 89, 36–47.
- Khan, M.I.; Siddique, R. Utilization of silica fume in concrete: Review of durability properties. Resour. Conserv. Recycl. 2011, 57, 30–35.
- Tam, V.W.; Gao, X.; Tam, C.M. Microstructural analysis of recycled aggregate concrete produced from two-stage mixing approach. Cem. Concr. Res. 2005, 35, 1195–1203.
- Sivamani, J.; Renganathan, N.T. Effect of fine recycled aggregate on the strength and durability properties of concrete modified through two-stage mixing approach. Environ. Sci. Pollut. Res. 2021.
- Zhang, J.; Wang, Y.; Xu, M.; Zhao, Q. Effect of carbon dioxide corrosion on compressive strength of oilwell cement. J. Chin. Ceram. Soc. 2009, 37, 642–647.
- Geng, J.; Sun, J. Characteristics of the carbonation resistance of recycled fine aggregate concrete. Constr. Build. Mater. 2013, 49, 814–820.
- Kaliyavaradhan, S.K.; Ling, T.-C. Potential of CO2 sequestration through construction and demolition (C&D) waste—An overview. J. CO2 Util. 2017, 20, 234–242.
- Liang, C.; Lu, N.; Ma, H.; Ma, Z.; Duan, Z. Carbonation behavior of recycled concrete with CO2 -curing recycled aggregate under various environments. J. CO2 Util. 2020, 39, 101185.
- Evangelista, L.; De Brito, J. Durability performance of concrete made with fine recycled concrete aggregates. Cem. Concr. Compos. 2010, 32, 9–14.
- Zhu, P.; Chen, K.; Hu, K. Carbonation Behavior of Repeated Recycled Fine Aggregate Concrete under Bending Load. KSCE J. Civ. Eng. 2019, 23, 729–736.
- Singh, N.; Singh, S. Validation of carbonation behavior of self compacting concrete made with recycled aggregates using microstructural and crystallization investigations. Eur. J. Environ. Civ. Eng. 2020, 24, 2187–2210.
- Evangelista, L.; De Brito, J. Durability of crushed fine recycled aggregate concrete assessed by permeability-related properties. Mag. Concr. Res. 2019, 71, 1142–1150.
- Sim, J.; Park, C. Compressive strength and resistance to chloride ion penetration and carbonation of recycled aggregate concrete with varying amount of fly ash and fine recycled aggregate. Waste Manag. 2011, 31, 2352–2360.
- Cartuxo, F.; De Brito, J.; Evangelista, L.; Jiménez, J.R.; Enrique, F.L. Increased durability of concrete made with fine recycled concrete aggregates using superplasticizers. Materials 2016, 9, 98.
- Debnath, B.; Li, M.; Liu, S.; Pan, T.; Ma, C.; Qiu, D. Melatonin-mediate acid rain stress tolerance mechanism through alteration of transcriptional factors and secondary metabolites gene expression in tomato. Ecotoxicol. Environ. Saf. 2020, 200, 110720.
- Berredjem, L.; Arabi, N.; Molez, L. Mechanical and durability properties of concrete based on recycled coarse and fine aggregates produced from demolished concrete. Constr. Build. Mater. 2020, 246, 118421.
- Salahuddin, H.; Qureshi, L.A.; Nawaz, A.; Razad, S.S. Effect of recycled fine aggregates on performance of Reactive Powder Concrete. Constr. Build. Mater. 2020, 243, 118223.
- Vigneshwari, M.; Arunachalam, K.; Angayarkanni, A. Replacement of silica fume with thermally treated rice husk ash in Reactive Powder Concrete. J. Clean. Prod. 2018, 188, 264–277.
- Kumar, B.V.; Ananthan, H.; Balaji, K. Experimental studies on utilization of recycled coarse and fine aggregates in high performance concrete mixes. Alex. Eng. J. 2018, 57, 1749–1759.
- Huang, J.; Zou, C.; Sun, D.; Yang, B.; Yan, J. Effect of recycled fine aggregates on alkali-activated slag concrete properties. Structures 2021, 30, 89–99.
- Omrane, M.; Kenai, S.; Kadri, E.-H.; Aït-Mokhtar, M. Performance and durability of self compacting concrete using recycled concrete aggregates and natural pozzolan. J. Clean. Prod. 2017, 165, 415–430.
More
