Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Mariana Levkiv.
Dental biomaterials were improved by nanotechnology. It manufactures better materials or improves the existing ones and forms the basis of novel methods for disease diagnosis and prevention. Modern nanotechnology makes oral health care services more acceptable for patients. Nanotechnology is now important area of research, covering a broad range of applications in dentistry.
- dentistry
- nanomedicine
- nanoparticle
1. Conservative Nano-Dentistry
1.1. Dentin/Tooth Hypersensitivity
Dentin/tooth hypersensitivity is a common condition that manifests as intense pain of short duration in response to external stimuli.
According to the “hydrodynamic theory” proposed by M. Brannstrom and A. Astron in 1964, the presence of damage to the enamel and/or cement in the cervical area with subsequent exposure of dentinal tubules in response to certain stimuli, can cause movement of dentinal fluid inside the tubules, indirectly stimulating the pulpal nerve endings, causing pain [24][1].
At the macroscopic level, hypersensitive dentin is no different to healthy dentin. Histologically, hypersensitive dentin has dilated dentinal tubules that can be twice as wide as in normal dentin. In addition, there is an increased number of dentinal tubules per unit area compared to normal dentin. As a result of doubling the diameter of the dentinal tubules, the fluid flow increases [25][2].
The main methods of treatment of dental hyperesthesia are based on the relief of the hydrodynamic mechanism, i.e., on the reduction in fluid movement in the dentinal tubules in response to external stimuli. This can be achieved by blockage of the microspaces with desensitizers; reducing the volume of microspaces with mineralizing agents [26][3].
1.2. Tooth Restoration Procedures with Nanomaterials
Nanocomposites
The major problem of resin composites is that more biofilms and plaques can be accumulated on their surface, compared to other restorative materials. Nanotechnology can aid in the development of bioactive dental materials to reduce or modify the influence of caries-related bacteria. Nanomaterials provide superior antimicrobial activity and display better physical properties in comparison to conventional materials. Agents such as silver, zinc oxide, calcium phosphate, calcium fluoride, quaternary ammonium polyethylenimine, and nanohydroxyapatite and/or nanofluorohydroxyapatite are incorporated into restorative materials such as composite resins, glass ionomer cements, and adhesive systems [29][6].
The summary information of the nanoparticles listed above is given in Table 6 [29,30,31,32,33,34,35,36,37,38,39,40]1 [6][7][8][9][10][11][12][13][14][15][16][17].
Table 61.
Nanoparticles with antibacterial and remineralizing ability used in dental restorative materials.
| Name of Nanoparticle |
Advantages/Disadvantages | Mechanism of Co-Interaction |
Examples of Materials |
Reason for Introduction into Material |
|---|
| NAg (nanoparticles of silver) | Antibacterial/can alter color of tooth or restoration | A bactericidal effect is achieved by interactions with the peptidoglycan cell wall and the plasma membrane; silver ions prevent bacterial DNA replication by interacting with the exposed sulfhydryl groups in bacterial proteins | Composite resin; dental adhesives |
NAg and NZnO have been incorporated in dental materials to kill cariogenic microorganisms in the marginal gaps and on the material surfaces | ||||||
| NZnO (nano zinc oxide particle) | Antibacterial action against several types of microorganisms, including S. Mutans/no evidence about increased mechanical properties | Bactericidal effect is due to modified cell membrane activity and oxidative stress; these generate active oxygen species such as H | 2 | O | 2 | that inhibit growth of planktonic microbes | Composite resin | |||
| Quaternary ammonium polyethylenimine nanoparticles | An antibacterial agent is copolymerized with the resin by forming a covalent bond with the polymer network, and therefore is immobilized in the composite and not released or lost over time | Cause bacterial lysis by binding to the cell membrane and causing cytoplasmic leakage | Composite resin; glassionomer cement |
Provide durable and permanent antibacterial capability to the dental material without significantly affecting the biologic balance in the oral cavity | ||||||
| Calcium phosphate nanoparticles | Remineralizing ability: can promote remineralization without loss of the mechanical characteristics of restorative material | Continuous release of calcium (Ca) and phosphate (PO | 4 | ) ions into oral environment increase the mineral content in the caries lesions | Composite resin; adhesive systems; glassionomer cement |
The presence of ACP (amorphous calcium phosphate) nanofillers (NACP) in dental composite resins is an approach to release calcium and phosphate ions continuously into the oral environment | ||||
| Calcium fluoride nanoparticles (CaF | 2 | ) | High fluoride release: caries-inhibiting effect without compromising on mechanical strength | Cumulative fluoride release increases with nano CaF | 2 | content, and resin composites containing 20–30% of CaF | 2 | nanoparticles have the same fluoride release rates as traditional and resin-modified glass ionomer materials | Composite resin | To inhibit cariogenic bacteria and reduce secondary caries rate |
| Nano hydroxyapatite and nano fluorohydroxyapatite (NHA, NFHA) | An increased resistance to demineralization when incorporated into glassionomer cement (GICs)/exceeded the clinically suitable maximum setting time when added into GICs | Has remineralization effect and biological compatibility of synthesized NHA; is used as substitute for the natural mineral constituent of dentin | Resin modified glassionomer | Remineralization rates with NFHA are higher than with micro bioactive glass particles |
One of the mainstreams of nanotechnology, which is widely used in dentistry, is incorporation of nanoparticles in composite materials. Composite resins are the most widely used dental materials for restoring dental cavities, especially because of their biomimetic ability [41][18]. Nanotechnologies make possible the emergence of materials with completely new characteristics—stronger, lighter, and thinner composite materials, and the quality of many materials can be improved using nanoparticles. Combining these properties in one material has led to the creation of a new class of materials, “nanocomposites”, in which nanomers and nanoclusters are used as fillers [42,43][19][20]. The benefits of “nanocomposites” are widely known to dentists. The rationale for use of nanoparticles includes the main indicators that assess the quality of composite materials, namely:
-
high strength, which allows them to be used for restoration and filling procedures on anterior and posterior teeth;
-
aesthetics, in particular the ability to manipulate the color shade of restorations in a wide range of values, as well as obtaining a stable shine;
-
minimal polymerization shrinkage that would help avoid marginal leakage problems as it is the main reason for secondary caries progression.
It is assumed that Indian American chemist Sumita Mitra is responsible for the introduction of nanoparticles in dentistry [44][21]. A composite material Filtek Supreme XT (3M ESPE) came out from under her microscope, which the American publisher “The Dental Advisor” recognized as a composite of the year for several years in a row together with Vitremer ™ triple-cured glass ionomer cement.
The fact is that the traditional material for filling teeth consists of an inorganic filler based on silicon and an organic matrix (resin), and such materials are not universal, they do not combine high strength with aesthetics [45][22]. This means that if the material is invisible, shines like your own teeth, it can only be used on the anterior teeth. Moreover, if the material is strong, does not wear out, it loses its gloss very quickly and turns yellow.
Nanocomposite Filtek ™ Supreme ™ XT solved this problem with its appearance. The innovative zirconium-silicon filler sintered in a special furnace. This unique filler adds strength and self-polishing to the material. The material can be used on the anterior and posterior teeth, achieving at the same time durable, completely invisible, aesthetic restorations of extremely high quality. That is why the material has gained popularity among experts.
It is claimed that Premise, Kerr/Sybron, Orange, CA builds on the proven success of Point 4 ™ Dental Hybrid Composite from Kerr, by using the same 0.4 micron barium glass filler, which provides outstanding blending—making restorations hard to detect. Benefits of this material come from the tri-modal filler system which uses three distinct filler sizes to increase loading. In addition, the incorporation of PPF (pre-polymerized filler) limits shrinkage and enhances polish ability and wear resistance [46][23].
The aesthetic properties of nanomaterials are superior to those in traditional composites, primarily due to the optical properties of nanoparticles, as well as better polishing and preservation of the polished surface for a long time. Nanofilled composite materials exhibit better physical properties, including compressive strength, tensile strength, toughness, flexural strength and abrasion resistance, etc., compared to microparticle-filled materials [47][24]. Therefore, nanocomposite materials are a real breakthrough and revolution in the history of the dental industry. Figure 12 gives a brief idea of the timeline of composite resins/adhesives development.
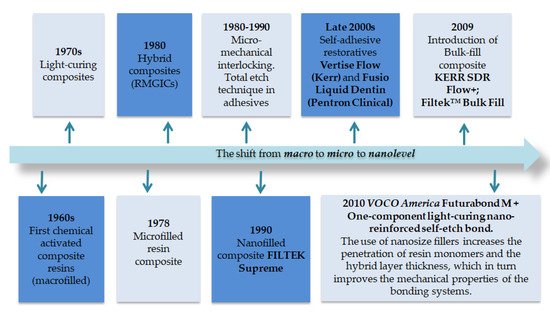
Figure 12.
Timeline of composite resins and adhesives including the nano era.
Adhesives
The development of dental adhesive systems has played a significant role in restorative dentistry, paving the way for minimal invasive dentistry. By using adhesives, dentists can undertake more conservative cavity design, preserving healthy tissue. Bonding of composite materials with tooth tissues is carried out using various systems of adhesive resins [48,49][25][26]. The resin–dentin bond depends on the infiltration of the adhesive system into the collagen matrix of the dentin, which is exposed through acid conditioning by creating a hybrid layer. In 2010, VOCO America (a subsidiary of VOCO GmbH, Cuxhaven, Germany) was the first to introduce nanoreinforced bonding agents. In this scenario, the use of nanosized fillers increases the penetration of resin monomers and the hybrid layer thickness, which in turn improves the mechanical properties of the bonding systems: i.e., Futurabond M VOCO tolerates residual moisture due to its hydrophilic properties that is beneficial in cases of poor moisture control. Integration of nanotechnologies in adhesive systems facilitates the micro shear bond strength as well [50][27].
Nanoreinforced adhesive Adper™ Single Bond Plus Adhesive give dentists confidence that the adhesive is perfectly mixed every time due to highly dispersed bonded nanofiller which does not allow particles to cluster together. The particles are stable and will not settle out of dispersion. Therefore, unlike some filled adhesives, Adper Single Bond Plus Adhesive does not require shaking prior to use [51][28].
Glass Ionomer Cement
Glass Ionomer Cement (GIC) originated in the middle of the 20th century, as a biocompatible, cost effective, tooth-colored restorative material and is constantly evolving. Considering its unique ability to bond to the tooth structure without the use of any bonding agent coupled with fluoride releasing potential, GIC has gradually emerged as the material of choice for various applications in the field of dentistry [52][29].
The glass ionomers with nanoparticles are called nanoionomers. The glass ionomer cement is widely used on the basis of its chemical binding to the tooth surface. Nanomers and nanoclusters are added to fluoroaluminosilicate glass [53][30]. The nanoionomer produces aesthetic and fluoride releasing properties. The nanoglass ionomer has high translucency and optical properties compared to the conventional GIC [54][31].
Ketac N-100 is the first resin-modified glass ionomer cement designed based on the nano-filler technology. Ketac N-100 nanoionomer represents a combination of the fluoraluminosilicate technology and the concept of nanotechnology encountered in FiltekTM Supreme Universal Restorative preparation [55][32].
Incorporation of nano-sized particles in powder-modified nanoglass ionomers improves their mechanical properties [56][33]. For the first time, it was assumed by De Caluwé et al. that doping conventional GICs with nano-sized glass particles can decrease the setting time and enhance the compression strength and elastic-modulus [57][34].
1.3. Nanotechnology in Endodontic Sealers
In endodontic practice, the problem of managing bacterial biofilm is extremely important. Persistent in the lumen of numerous dentinal tubules, the microflora is virtually invulnerable to medical and instrumental treatment of root canals. The diameter of the dentinal tubules is only 200–300 nm and this prevents the penetration of even the strongest antiseptics. Incorporation of metallic nanoparticles into antiseptics for root canals can help to manage persistent microflora (Enterococcus faecalis) after one week [58,59][35][36].
Research by Leng, D. et al. (2020) showed the potential of using a mixture of calcium hydroxide paste and nanosilver for intracanal drug treatment [60][37].
The advantages of using nano endodontic sealers are obvious: they seal better in comparison to the conventional sealers and the use of nanoparticles serves as a good antimicrobial agent [61][38].
The application of nanotechnology in endodontics includes the insertion of bioceramic nanoparticles such as bioglass, zirconium, and glass ceramics in endodontic sealers. A nanomaterial sealer was recently developed on a bioceramic basis EndoSequence BC Sealer—Brasseler USA, consisting of nanosized particles of calcium silicate, calcium hydroxide, CaP. A feature of the material is the formation of complex nanocomposite structural particles of hydroxyapatite and calcium silicate during the hydration reaction in the root canal. The use of nanosized particles helps to easily deliver the material with an ultra-thin capillary needle size of 0.0012 mm. Nanodisperse materials provide excellent tightness and dimensional stability, excellent biocompatibility and bioactivity, excellent antimicrobial properties at high alkaline pH 12.8.
TotalFill BC Sealer (FKG, La Chaux-des-Fonds, Switzerland) and TotalFill BC Sealer HiFlow (FKG, La Chaux-des-Fonds, Switzerland) is a new class of endodontic sealers, with potential further benefits due to their bioactivity.
This study [62][39] indicates that both TotalFill BC sealer and TotalFill BC HiFlow are biocompatible and exhibit potential bioactivity. TotalFill® BC Sealer™ and BC Sealer HiFlow™ are the “state-of-the-art” in endodontic obturation.
GuttaFlow Bioseal (Coltene/Whaledent AG, Altstatten, Switzerland) is a silicon-based sealer containing polydimethylsiloxane, and a mixture of gutta-percha and calcium silicate particles [63][40]. It is composed of a unique mixture of finely ground gutta percha, RoekoSeal® root canal sealer and nano-silver. The manufacturing company claims that this sealer has excellent adaptation due to its optimal flowability and can undergo gradual volumetric expansion, thus, it can adapt well to the root canal walls and can be used not only as a sealer but even as a root filling material. The sealer part of GuttaFlow is highly thixotropic, has a fine grain size (<9 µm), and the material flows well under slight pressure into the lateral canals [64,65,66,67,68][41][42][43][44][45].
Gutta Percha points have been most widely used for years and established themselves as a gold standard in root canal obturation techniques. In addition, it has proved itself successful with different techniques of root canal sealing. Attempts have been made to improve optimum seal and therapeutic effects by addition of various materials including nanoparticles to gutta percha composition [69][46]. Nanodiamond coated gutta percha embedded with nanodiamond amoxicillin conjugates could reduce the likelihood of root canal reinfection and enhance the treatment outcomes. Antibacterial effects against various intracanal microorganisms are gained by coating standard gutta percha points with silver nanoparticles [70][47].
1.4. Nanotechnology in Periodontology
Chronic generalized periodontitis it is a mainly bacterial dependent disease together with other co-reasons. Due to long lasting inflammation, it can lead to loosening of teeth or tooth loss. The current treatment options include mechanical removal of pathogenic biofilms and providing some drug treatment, both local and systemic. Dental researchers attempted to generate an effective and satisfactory drug delivery system for the treatment of periodontal diseases that will be better than conventional ones. Current trends are the use of microparticles [71][48] and nano-based delivery systems [72,73][49][50].
Microspheres of “Arestin” (minocycline HCl), 1 mg, is a concentrated, locally applied antibiotic that remains active in the pocket for an extended period of time and reduces pocket depth [74][51]. The microspheres release antibiotic over time, targeting bacteria to reduce pocket depth, so gums can heal better than with scaling and root planing alone.
Nano-based delivery systems can find significant application for eliminating bacterial pathogens. The use of charged nanoparticles is extremely useful since many of the microorganisms are themselves charged. The charged nanoparticles could be used to directly affect the bacteria or alter the microenvironment of it [75,76,77][52][53][54].
2. Nanotechnology in the Surgical Field
2.1. Nanoanesthesia
In the era of nano-dentistry, a colloidal suspension containing millions of active analgesic micron-size dental robots will be instilled on the patient’s gingiva. After contacting the surface of crown or mucosa, the ambulating nanorobots reach the pulp via the gingival sulcus, lamina propria, and dentinal tubules guided by combination of chemical gradients, temperature differentials, and even positional navigation all under the control of the on-board nanocomputer that is controlled by the dentist [78][55].
Once installed in the pulp, the analgesic dental robots may be controlled by the dentist to shut down all sensitivity in any particular tooth that requires treatment. After oral procedures are completed, the dentist orders the nanorobots to restore all sensation, to relinquish control of nerve traffic, and to egress from the tooth by similar pathways used for ingress [79][56].
2.2. Nanotechnology in Dental Implants
Structural and functional fusion of the surface of the dental implant with the surrounding bone (osteointegration) is crucial for short-term and long-term results. Titanium dental implants have been used successfully for the past 30 years, but they still have disadvantages due to their full osseointegration and the fact that their mechanical properties do not match the properties of the bones [80][57].
Advances in the production of nanoparticles for implant surface coating and nano formatting of dental implants lead to better osseointegration and improved physiological functions of implants [81][58]. The application of nanotechnology on the surfaces of dental implants has many different mechanisms. In particular, the surfaces can potentially take an organized (isotropic) or unorganized (anisotropic) pattern. Due to the difficulty of applying standardized sequences to complex structures, the template for dental implants is usually anisotropic. Titanium dioxide (TiO2) nanoparticles have been explored in recent years as antimicrobial agents [82][59].
A wide variety of methods are used to create nano-features on the surface of dental implants. They can be divided into chemical (anodic oxidation, combinations of acids (bases) and oxidants) and physical (plasma spray, blasting) [83,84][60][61].
Antimicrobial peptides (AMPs), such as LL37 peptides, may be immobilized on the surface of medical devices, as dental implants, to render them with antimicrobial and angiogenic properties.
Both soluble and immobilized LL37 peptides have potent antimicrobial activity against Gram-positive and Gram-negative bacteria in the presence of 10% human serum (HS). However, the immobilized LL37 peptides showed less cytotoxicity to endothelial cells (ECs) at a concentration that was able to kill bacteria [85][62].
2.3. Nanotechnologies in the Correction of Deformations and Defects of Bones
Nanophase materials have shown promising results in the treatment of various deformities and bone defects. Nanophase hydroxyapatite and nanophase carbon are the two main promising types of nanophase materials used to treat bone defects.
Nanophase hydroxyapatite shows excellent osteoblastic adhesion compared to traditional materials. Nanoparticles of nanophase hydroxyapatite (HA) are used to treat bone defects—NanOSSTM HA (Angstrom Medica, Woburn, MA, USA), Vitosso (Orthovita, Inc, Malvern, PA, USA) HA + tricalcium phosphate (tri CaP), and Ostim HA (Osartis GmbH, Germany) [86,87][63][64].
Unlike nanophase hydroxyapatite, carbon nanophase exhibits excellent biomechanical properties due to a combination of not only nanoscale but also similar to natural HA. Thus, making it the material with the greatest potential for the correction of maxillofacial defects and maxillofacial implant material in the future.
3. Prosthetic Dentistry
Another area where nanotechnology has been used is the use of submicron grain sized ceramics for the production of all-ceramic restorations. The logic for using nanometer-sized powders for the production of ceramic monoliths is esthetics, wear properties, and for maximizing the strength of the ceramic.
It has been established that nanoglass ceramics cause less abrasion of antagonist enamel than ordinary facing ceramics; in addition, nanoglass ceramics have demonstrated high strength under bending loads [88,89,90][65][66][67].
The addition of silver nanoparticles to the polymer for the base of the denture has shown positive results in the treatment of stomatitis associated with wearing dentures. In this regard, scientists have concluded that the addition of silver nanoparticles to dentures helps prevent infections of the oral mucosa.
4. Preventive Nano-Dentistry
The goal of modern dentistry is to prevent rather than treat biofilm-dependent oral diseases, i.e., dental caries, endodontic and periodontal diseases. Nanotechnology offers new approaches for preventive measures in oral diseases, particularly dental caries and periodontal diseases [94][71].
Prevention of caries is one of the main methods that reduces the prevalence of this disease. Caries prevention methods are constantly improving, but still the most affordable tool is the use of therapeutic and prophylactic toothpastes. With the development of nanotechnology in dentistry, now even daily brushing can ensure hygiene and protection of the oral cavity at the nano level.
Researchers have developed a nano-toothbrush. Including colloidal particles of nanogold or nanosilver between the bristles of a toothbrush [95,96][72][73] can lead to a significant reduction in periodontal disease.
Oral hygiene products, such as toothpastes and mouthwashes, have also been nanomodified according to recent reports [97][74].
For example, nanocalcium fluoride, which is part of mouthwashes, reduces the activity of caries, reduces dentin permeability, and increases the labile concentration of fluoride in oral fluid [98][75]. Toothpastes containing calcium carbonate nanoparticles and 3% nanosized sodium trimetaphosphate promote remineralization of early carious lesions compared to conventional toothpaste without nano-additives [99][76]. According to the results of an in vitro study, toothpastes containing nano hydroxyapatite (NHA) crystals significantly increased the value of microhardness in human enamel after erosive influence, compared to the same toothpaste without NHA [100][77].
The higher reparative capacity of nanomaterials compared to the same material on a micro or macro-level may be due to the fact that inorganic building blocks in enamel have a size of 20–40 nm, which makes it logical to assume a higher affinity for nanosized particles. Remineralizing toothpaste with peptide complex and nano-hydroxyapatite Vivax Dent strengthens tooth enamel, prevents leaching of calcium and phosphorus from bone tissue, fights bacteria and plaque, and prevents caries and odor. It is suitable for the treatment of stomatitis, periodontitis, and other inflammatory processes of the oral cavity, as well as for the prevention of caries.
5. Nanotechnologies in Diagnosis
Nanoparticles are used not only for the treatment and prevention of dental diseases, but also as agents for extra research methods. Thus, more recently, the latest word in dental technology was “laser diagnosis” of caries, based on the optical phenomenon of transillumination. Researchers from the University of Michigan [102][79] used fluorescent dioxide-labeled cations of 150 nm starch nanoparticles. When irrigating the oral cavity with a solution of such particles, the latter easily penetrated into the micropores of the foci of demineralization, which were then easily detected under the light of a standard light curing halogen lamp. The technology described in 2017 allows detection of actively occurring superficial caries in the early stages of pathogenesis.
Molecular Imaging
Optical coherence tomography (OCT) it is a direct simulation of ultrasound, that is based on low-coherence interferometry, typically employing near-infrared light. OCT shows enormous potential for its application in more effective diagnosis and therapy of caries and erosive tooth wear. This technique is advantageous in qualitative assessment of pit and fissure sealing as well as providing great potential for imaging the outline form of the pulp chamber: pulp horns can be visualized during dental procedures and accidental pulp exposure can be avoided [103][80].
Nanotechnology is a useful tool for cancer detection and disease monitoring, and also increases the possibility of specific targeted cancer therapy [104][81].
OCT is used not only in caries diagnostics but also for oral cancer screening and monitoring. The main type of malignant neoplasm of the oral cavity is a squamous cell carcinoma of the oral cavity, which accounts for more than 90% of all cancers of the oral cavity. Squamous cell carcinoma is an aggressive cancer that has a poor prognosis and a high recurrence rate, which can even lead to death.
With the development of nanotechnology, different types of nanoparticles came into use as specific contrast agents for magnetic resonance imaging, optical coherence tomography, etc. Shanavas et al. created a nanoagent that is a combination of folate preconjugated chitosan and poly (magnetic lactide with glycolide) nanoparticles for simultaneous cancer therapy and a contrast agent for magnetic resonance imaging at the same time. Oral cancer cells with positive folic acid receptors showed increased uptake of nanoparticles and caused a significant increase in cytotoxicity [105][82].
The gold nanoparticles are promising contrast agents for OCT due to biocompatibility; they can provide localized surface plasmon resonances at wavelengths of near-infrared radiation, which avoids the predominant absorption in tissues [106][83].
Photoacoustic (PA) imaging is a new non-invasive technique of optical diagnostic technology. Near-infrared absorbing organic nanoparticles are used in PA imaging applications. Photoacoustic waves generated by the laser pulse are then converted into photoacoustic images. Photoacoustic images have improved image depth in comparison to conventional optical images. Organic nanoparticles with their exclusive benefits can play an important role in advancing PA molecular imaging in preclinical investigation and clinical use [107][84].
The nano-based single biomarker method is used to detect oral cancer [108][85]. Investigation of tumor molecular biomarkers—such as tumor necrosis factor-alpha (TNF-α), vascular endothelial growth factor, and interleukin 6 (IL 6)—gives great promise for early cancer detection. The research revealed TNF-α by the method of gold protein chips using fluorescence microscopy of complete internal reflection [109][86].
Despite of a wide range of benefits of nanoparticle application, there is a disadvantage of cytotoxicity that is seen only in higher concentrations. The cytotoxicity depends on the size—selective difference [110,111,112][87][88][89]. Therefore, new studies aimed at producing safe and biocompatible materials are needed [113,114][90][91].
References
- Onwubu, S.C.; Mdluli, P.S.; Singh, S. The Effectiveness of Nanomaterials in the Management of Dentine Hypersensitivity-A review. J. Clin. Rev. Case Rep. 2018, 3, 1–5.
- Olley, R.C.; Sehmi, H. The rise of dentine hypersensitivity and tooth wear in an ageing population. Br. Dent. J. 2017, 223, 293–297.
- Ogihara, T.; Tomiyama, K.; Iizuka, J.; Ishizawa, M.; Shiiya, T.; Mukai, Y. Effects of desensitizer containing fluoroaluminocalciumsilicate glass nanoparticles on remineralization of root dentin subsurface lesions in vitro. Dent. Mater. J. 2021, 40, 1027–1032.
- Mitthra, S.; Karthick, A.; Anuradha, B.; Mensudar, R.; Sadhana, K.R.; Varshini, G.N. Nanorobots–A small wonder. Biosci. Biotechnol. Res. 2016, 13, 2131–2134.
- Şuhani, M.F.; Băciuţ, G.; Băciuţ, M.; Şuhani, R.; Bran, S. Current perspectives regarding the application and incorporation of silver nanoparticles into dental biomaterials. Clujul Med. 2018, 91, 274–279.
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.; Melo, M. Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioact. Mater. 2018, 4, 43–55.
- Al-Dulaijan, Y.A.; Cheng, L.; Weir, M.D.; Melo, M.A.S.; Liu, H.; Oates, T.W.; Xu, H.H. Novel rechargeable calcium phosphate nanocomposite with antibacterial activity to suppress biofilm acids and dental caries. J. Dent. 2018, 72, 44–52.
- Iftikhar, S.; Jahanzeb, N.; Saleem, M.; Rehman, S.; Matinlinna, J.P.; Khan, A.S. The trends of dental biomaterials research and future directions: A mapping review. Saudi Dent. J. 2021, 33, 229.
- Alsuraifi, A. Metallic Nanoparticles in Dental Biomaterials: A review. AAJMS 2020, 3, 27–37.
- Nirubama, K.; Rajeshkumar, S. Enhanced antibacterial activity of silver nanoparticles synthesised using symplocos racemosa. Int. J. Pharm. 2020, 11, 4120–4125.
- Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020, 15, 819–839.
- Raorane, D.; Pednekar, S.; Dashaputra, R. Dental Applications of Nanotechnology; Chaughule, R.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2018.
- Beyth, N.; Yudovin-Farber, I.; Basu, A.; Weiss, E.I.; Domb, A.J. Antimicrobial nanoparticles in restorative composites. In Emerging Nanotechnologies in Dentistry; William Andrew Publishing: Norwich, UK, 2018; pp. 41–58.
- Song, W.; Ge, S. Application of antimicrobial nanoparticles in dentistry. Molecules 2019, 24, 1033.
- Noori, A.J.; Kareem, F.A. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon 2019, 5, e02568.
- Santoso, J.; Purbiati, M. Antibacterial activity of silver nanoparticles on fixed retainer adhesive toward treponema denticola. Int. J. Appl. Pharm. 2019, 11, 198–200.
- Prabha, R.D.; Kandasamy, R.; Sivaraman, U.S.; Nandkumar, M.A.; Nair, P.D. Antibacterial nanosilver coated orthodontic bands with potential implications in dentistry. Indian J. Med. Res. 2016, 144, 580.
- Freitas, F.; Pinheiro de Melo, T.; Delgado, A.H.; Monteiro, P.; Rua, J.; Proença, L.; Mendes, J.J. Varying the Polishing Protocol Influences the Color Stability and Surface Roughness of Bulk-Fill Resin-Based Composites. J. Funct. Biomater. 2021, 12, 1.
- Lee, J.H.; Seo, S.J.; Kim, H.W. Bioactive glass-based nanocomposites for personalized dental tissue regeneration. Dent. Mater. J. 2016, 35, 710–720.
- Lopes, I.A.D.; Monteiro, P.J.V.C.; Mendes, J.J.B.; Gonçalves, J.M.R.; Caldeira, F.J.F. The effect of different finishing and polishing techniques on surface roughness and gloss of two nanocomposites. Saudi Dent. J. 2018, 30, 197–207.
- Mitra, S.B. Nanoparticles for Dental Materials: Synthesis, Analysis, and Applications. In Emerging Nanotechnologies in Dentistry; William Andrew Publishing: Norwich, UK, 2018; pp. 17–39.
- Pieniak, D.; Walczak, A.; Walczak, M.; Przystupa, K.; Niewczas, A.M. Hardness and wear resistance of dental biomedical nanomaterials in a humid environment with non-stationary temperatures. Materials 2020, 13, 1255.
- Panchbhai, A. Nanocomposites: Past, Present, and Future of Dentistry. In Applications of Nanocomposite Materials in Dentistry; Woodhead Publishing: Cambridge, UK, 2019; pp. 181–190.
- Subramani, K.; Ahmed, W. (Eds.) Emerging Nanotechnologies in Dentistry; William Andrew: Norwich, NY, USA, 2017.
- Nassif, M.; El Askary, F. Nanotechnology and Nanoparticles in Contemporary Dental Adhesives. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–198.
- Carvalho, E.V.; De Paula, D.M.; Neto, D.A.; Costa, L.S.; Dias, D.F.; Feitosa, V.P.; Fechine, P.B.A. Radiopacity and mechanical properties of dental adhesives with strontium hydroxyapatite nanofillers. J. Mech. Behav. Biomed. 2020, 101, 103447.
- Althomali, Y.M.; Ebrahim, M.I. Microshear bond strength of Nano-Bond adhesive containing nanosized aluminum trioxide particles. J. Orthod. Sci. 2017, 6, 71–75.
- 3M ESPE. Available online: https://multimedia.3m.com/mws/media/546458O/3m-espe-adhesive-technology-review.pdf (accessed on 22 October 2021).
- Sajjad, A.; Bakar, W.Z.W.; Mohamad, D.; Kannand, T.P. Characterization and efficacy of fluoride elusion of a novel glass ionomer nano zirconia silica hydroxyapatite hybrid material. Fluoride 2019, 52, 507–516.
- Mirsasaani, S.S.; Hemati, M.; Dehkord, E.S.; Yazdi, G.T.; Poshtiri, D.A. Nanotechnology and nanobiomaterials in dentistry. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 19–37.
- Hannig, M.; Hannig, C. Nanobiomaterials in preventive dentistry. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–223.
- Vasiliu, S.; Racovita, S.; Gugoasa, I.A.; Lungan, M.A.; Popa, M.; Desbrieres, J. The Benefits of Smart Nanoparticles in Dental Applications. Int. J. Mol. Sci. 2021, 22, 2585.
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Rehman, I.U. Modifications in glass ionomer cements: Nano-sized fillers and bioactive nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134.
- AlOtaibi, G. Recent advancements in glass ionomer materials with introduction of nanotechnology: A review. Int J Oral Dent Health. 2019, 7, 21.
- Loyola-Rodríguez, J.P.; Torres-Méndez, F.; Espinosa-Cristobal, L.F.; García-Cortes, J.O.; Loyola-Leyva, A.; González, F.J.; Contreras-Palma, G. Antimicrobial activity of endodontic sealers and medications containing chitosan and silver nanoparticles against Enterococcus faecalis. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019851771.
- Ioannidis, K.; Niazi, S.; Mylonas, P.; Mannocci, F.; Deb, S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019, 35, 1614–1629.
- Leng, D.; Li, Y.; Zhu, J.; Liang, R.; Zhang, C.; Zhou, Y.; Li, J. The Antibiofilm activity and mechanism of nanosilver-and nanozinc-incorporated mesoporous calcium-silicate nanoparticles. Int. J. Nanomed. 2020, 15, 3921.
- Gonzalez-Luna, I.P.; Martínez-Castañón, G.A.; Zavala-Alonso, N.V.; Patiño-Marin, N.; Niño-Martínez, N.; Morán-Martínez, J.; Ramírez-González, J.H. Bactericide effect of silver nanoparticles as a final irrigation agent in endodontics on Enterococcus faecalis: An ex vivo study. J. Nanomater. 2016, 2016, 7597295.
- Santos, J.M.; Coelho, C.M.; Sequeira, D.B.; Marques, J.A.; Pereira, J.F.; Sousa, V.; Palma, P.J.; Santos, A.C. Subcutaneous implantation assessment of new calcium-silicate based sealer for warm obturation. Biomedicines 2021, 9, 24.
- Gandolfi, M.G.; Siboni, F.; Prati, C. Properties of a novel polysiloxane-guttapercha calcium silicate-bioglass-containing root canal sealer. Dent. Mater. 2016, 32, 113–126.
- Dastorani, M.; Malekpour, B.; AminSobhani, M.; Alemrajabi, M.; Mahdian, A.; Malekpour, B. Comparison of bacterial microleakage of three bioactive endodontic sealers in simulated underwater diving and aviation conditions. BMC Oral Health 2021, 21, 345.
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562.
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle technology and its implications in endodontics: A review. Biomater. Res. 2020, 24, 21.
- Wong, J.; Zou, T.; Lee, A.; Zhang, C. The Potential Translational Applications of Nanoparticles in Endodontics. Int. J. Nanomed. 2021, 16, 2087–2106.
- Zakrzewski, W.; Dobrzyński, M.; Zawadzka-Knefel, A.; Lubojański, A.; Dobrzyński, W.; Janecki, M.; Kurek, K.; Szymonowicz, M.; Wiglusz, R.J.; Rybak, Z. Nanomaterials application in endodontics. Materials 2021, 14, 5296.
- Verma, S.; Chandra, A.; Jena, A.; Sharan, J. Nanotechnology in Endodontics: A Hope or Hype. Trends Biomater. Artif. Organs 2021, 35, 190–202.
- Vishwanath, V.; Rao, H.M. Gutta-percha in endodontics-A comprehensive review of material science. J. Conserv. Dent. 2019, 22, 216–222.
- Tan, O.L.; Safii, S.H.; Razali, M. Commercial local pharmacotherapeutics and adjunctive agents for nonsurgical treatment of periodontitis: A contemporary review of clinical efficacies and challenges. Antibiotics 2020, 9, 11.
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent Mater. 2020, 36, 1365–1378.
- Liang, J.; Peng, X.; Zhou, X.; Zou, J.; Cheng, L. Emerging applications of drug delivery systems in oral infectious diseases prevention and treatment. Molecules 2020, 25, 516.
- Patel, S.K.; Greene, A.C.; Desai, S.M.; Rothstein, S.; Basha, I.T.; MacPherson, J.S.; Rohan, L.C. Biorelevant and screening dissolution methods for minocycline hydrochloride microspheres intended for periodontal administration. Int. J. Pharm. 2021, 596, 120261.
- Kishen, A. Nanotechnology in Endodontics; Springer International: Cham, Switzerland, 2016.
- Mok, Z.H.; Proctor, G.; Thanou, M. Emerging nanomaterials for dental treatments. Emerg. Top. Life Sci. 2020, 4, 613–625.
- Carter, S.D.; Costa, P.F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Malda, J. Additive Biomanufacturing: An Advanced Approach for Periodontal Tissue Regeneration. Ann. Biomed. Eng. 2017, 45, 12–22.
- Bordoloi, P.; Shahira, S.; Ramesh, A.; Thomas, B. Nanorobotic wonders: A revolutionary era in periodontics. Indian J. Multidiscip. Dent. 2018, 8, 101.
- Mazumder, S.; Biswas, G.R.; Majee, S.B. Applications of Nanorobots in Medical Techniques. Int. J. Pharm. Sci. Res. 2020, 11, 3138–3147.
- Subramani, K.; Mathew, R.T.; Pachauri, P. Titanium surface modification techniques for dental implants—from microscale to nanoscale. In Emerging Nanotechnologies in Dentistry, 2nd ed.; William Andrew Publishing: Norwich, UK, 2018; pp. 99–124.
- Jadhav, K.; Rajeshwari, H.R.; Deshpande, S.; Jagwani, S.; Dhamecha, D.; Jalalpure, S.; Baheti, D. Phytosynthesis of gold nanoparticles: Characterization, biocompatibility, and evaluation of its osteoinductive potential for application in implant dentistry. Mater. Sci. Eng. C 2018, 93, 664–670.
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54.
- Subramani, K.; Lavenus, S.; Rozé, J.; Louarn, G.; Layrolle, P. Impact of nanotechnology on dental implants. In Emerging Nanotechnologies in Dentistry; Springer: Berlin/Heidelberg, Germany, 2018; pp. 83–97.
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338.
- Comune, M.; Rai, A.; Palma, P.; TondaTuro, C.; Ferreira, L. Antimicrobial and pro-angiogenic properties of soluble and nanoparticle-immobilized LL37 peptides. Biomater. Sci. 2021, 9, 8153–8159.
- Nandagopal, N.; Usha, M.; Sreejith, S.; Rajan, S. A clinical review of nanotechnology in maxillofacial practice. J. Oral Res. Rev. 2021, 13, 149–160.
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug Delivery (Nano) Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv. Sci. 2021, 8, 2004014.
- Azari, A.; Nikzad, S.; Yazdani, A.; Atri, F.; Anvari-Yazdi, A.F. Deposition of crystalline hydroxyapatite nano-particle on zirconia ceramic: A potential solution for the poor bonding characteristic of zirconia ceramics to resin cement. J. Mater. Sci. Mate.r Med. 2017, 28, 111.
- Uskoković, V.; Abuna, G.; Ferreira, P.; Wu, V.M.; Gower, L.; Pires-de-Souza, F.C.P.; Geraldeli, S. Synthesis and characterization of nanoparticulate niobium-and zinc-doped bioglass-ceramic/chitosan hybrids for dental applications. J. Sol-Gel Sci. Technol. 2021, 97, 245–258.
- Abd Alwahab, S.; Moosa, J.M.; Muafaq, S. Studying the Influence of Nano ZnO and Nano ZrO2 Additives on Properties of PMMA Denture Base. Indian J. Public Health Res. Dev. 2020, 11, 2047–2051.
- Domagała, I.; Przystupa, K.; Firlej, M.; Pieniak, D.; Gil, L.; Borucka, A.; Levkiv, M. Analysis of the Statistical Comparability of the Hardness and Wear of Polymeric Materials for Orthodontic Applications. Materials 2021, 14, 2925.
- Wang, R.; Kayacan, R.; Küçükeşmen, C. Nanotubes/polymethyl methacrylate composite resins as denture base materials. In Carbon Nanomaterials for Biomedical Applications; Springer: Cham, Switzerland, 2016; pp. 227–240.
- Zakrzewski, W.; Dobrzynski, M.; Dobrzynski, W.; Zawadzka-Knefel, A.; Janecki, M.; Kurek, K.; Lubojanski, A.; Szymonowicz, M.; Rybak, Z.; Wiglusz, R.J. Nanomaterials Application in Orthodontics. Nanomaterials 2021, 11, 337.
- Zhou, L.; Wong, H.M.; Li, Q.L. Anti-Biofouling Coatings on the Tooth Surface and Hydroxyapatite. Int. J. Nanomed. 2020, 15, 8963.
- Raval, C.; Vyas, K.; Gandhi, U.; Patel, B.; Patel, P. Nanotechnology in dentistry: A review. J. Adv. Med. Dent. Sci. Res. 2016, 4, 51–53.
- AlKahtani, R.N. The implications and applications of nanotechnology in dentistry: A review. Saudi Dent. J. 2018, 30, 107–116.
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485.
- Al-Ajely, M.S.; Ziadan, K.M.; Al-Bader, R.M. Preparation and characterization of calcium fluoride nano particles for dental applications. Int. J. Res. Granthaalayah 2018, 6, 338–346.
- Emerenciano, N.G.; Delbem, A.C.B.; Pessan, J.P.; Nunes, G.P.; Neto, F.N.S.; de Camargo, E.R.; Danelon, M. In situ effect of fluoride toothpaste supplemented with nano-sized sodium trimetaphosphate on enamel demineralization prevention and biofilm composition. Arch. Oral Biol. 2018, 96, 223–229.
- Ebadifar, A.; Nomani, M.; Fatemi, S.A. Effect of nano-hydroxyapatite toothpaste on microhardness of artificial carious lesions created on extracted teeth. J. Dent. Res. Den.t Clin. Dent. Prospect. 2017, 11, 14–17.
- Ahmed, F.; Prashanth, S.T.; Sindhu, K.; Nayak, A.; Chaturvedi, S. Antimicrobial efficacy of nanosilver and chitosan against Streptococcus mutans, as an ingredient of toothpaste formulation: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2019, 37, 46–54.
- Jones, N.A.; Chang, S.Y.; Troske, W.J.; Clarkson, B.H.; Lahann, J. Carious Lesions: Nanoparticle-Based Targeting and Detection of Microcavities. Adv. Healthc. Mater. 2017, 6, 1600883.
- Schneider, H.; Park, K.J.; Häfer, M.; Rüger, C.; Schmalz, G.; Krause, F.; Haak, R. Dental applications of optical coherence tomography (OCT) in cariology. Appl. Sci. 2017, 7, 472.
- Lin, B.; Wu, J.; Wang, Y.; Sun, S.; Yuan, Y.; Tao, X.; Lv, R. Peptide functionalized upconversion/NIR II luminescent nanoparticles for targeted imaging and therapy of oral squamous cell carcinoma. Biomater. Sci. 2021, 9, 1000–1007.
- Shanavas, A.; Sasidharan, S.; Bahadur, D.; Srivastava, R. Magnetic core-shell hybrid nanoparticles for receptor targeted anti-cancer therapy and magnetic resonance imaging. J. Colloid Interface Sci. 2017, 486, 112–120.
- van Manen, L.; Dijkstra, J.; Boccara, C.; Benoit, E.; Vahrmeijer, A.L.; Gora, M.J.; Mieog, J.S.D. The clinical usefulness of optical coherence tomography during cancer interventions. J. Cancer Res. Clin. Oncol. 2018, 144, 1967–1990.
- Jiang, Y.; Pu, K. Advanced photoacoustic imaging applications of near-infrared absorbing organic nanoparticles. Small 2017, 13, 1700710.
- Chen, X.J.; Zhang, X.Q.; Liu, Q.; Zhang, J.; Zhou, G. Nanotechnology: A promising method for oral cancer detection and diagnosis. J. Nanobiotechnol. 2018, 16, 52.
- Abram, T.J.; Floriano, P.N.; Christodoulides, N.; James, R.; Kerr, A.R.; Thornhill, M.H.; McDevitt, J.T. ‘Cytology-on-a-chip’based sensors for monitoring of potentially malignant oral lesions. Oral Oncol. 2016, 60, 103–111.
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J. Drug Deliv. Sci. Technol. 2020, 61, 102316.
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449.
- Sahu, D.; Kannan, G.M.; Tailang, M.; Vijayaraghavan, R. In vitro cytotoxicity of nanoparticles: A comparison between particle size and cell type. J. Nanosci. 2016, 2016, 1–9.
- Gracco, A.; Siviero, L.; Dandrea, M.; Crivellin, G. Use of nanotechnology for the superlubrication of orthodontic wires. In Nanobiomaterials in Dentistry: Applications of Nanobiomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 11, pp. 241–267.
- Totu, E.E.; Cristache, C.M.; Perieanu, V.S.; Burlibasa, M.; Petre, D.C.; Burlibasa, L. Are Nano TiO2 Inclusions Improving Biocompatibility of Photocurable Polydimethylsiloxane for Maxillofacial Prosthesis Manufacturing? Appl. Sci. 2021, 11, 3777.
More
