Molybdenum and its alloys, with high melting points, excellent corrosion resistance and high temperature creep resistance, are a vital high-temperature structural material. However, the poor oxidation resistance at high temperatures is a major barrier to their application.
- molybdenum alloys
- coating
- oxidation behavior
- microstructure
- high-temperature
1. Introduction
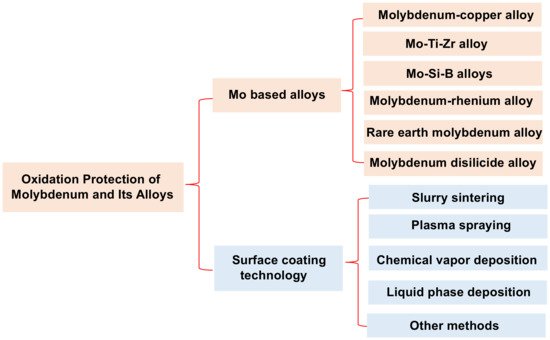
Molybdenum and molybdenum-based alloys have a high melting point (2620 °C), good high-temperature mechanical properties and high conductivity and thermal conductivity, and are widely used in high-temperature structures [5,6,7,8,9,10][1][2][3][4][5][6]. However, the alloys have a poor oxidation resistance, and the “Pesting oxidation” at 400–800 °C and oxidation decomposition above 1000 °C are the main factors that limit their application [11,12,13,14][7][8][9][10]. At present, the alloying and surface-coating technology are the main methods to increase the oxidation resistance of the basal materials [15,16][11][12]. The types of molybdenum alloys and the various surface coating technologies of Mo and its alloys are shown in Figure 1 [17,18,19,20][13][14][15][16]. It can been seen that the Ti, Zr, W, Re, Si, B, Hf, C and rare earth oxides are often added to pure Mo as beneficial elements to prepare molybdenum alloys. However, the result of alloying is not satisfactory when considering the mechanical properties and high-temperature oxidation resistance of the alloys [21,22][17][18]. For example, adding a certain amount Ti element to the alloy can enhance its strength, but it will further accelerate the oxidation of the alloy [23][19]. Mo–Si–B alloys have satisfactory high temperature oxidation resistance, but their fracture toughness is poor. Mo–Ti–Si–B alloys are considered as a promising ultra-high temperature material. However, their oxidation resistance and mechanical properties need to be further studied [24][20].
2. Microstructure and Oxidation Behavior of Coatings
2.1. Coatings Prepared by Slurry Sintering (SS)
2.1.1. Microstructure and Growth Mechanism of SS Coatings
2.1.2. Oxidation Behavior and Mechanism of SS Coatings
It is observed that an oxide layer forms on the surface of SS coatings after oxidation, which is mainly composed of SiO2, TiO2, Mo5Si3, etc. Compared with the original coating, the thickness of the oxidized coating increases significantly, which is due to the volume of the coating expanding and the interface migration caused by the inter-diffusion reaction. However, the thickness of the MoSi2 layer decreases significantly due to the growth of the oxide film and the migration of the interface layer. By contrast, the interdiffusion between the coating and the substrate becomes more sufficient with the increase of exposure time, resulting in a significant increase in the thickness of the interface layer dominated by Mo5Si3 [43,44,45,46][23][24][25][26].2.2. Coatings Prepared by Plasma-Spraying Technique
2.2.1. Microstructure and Growth Mechanism of Plasma-Spraying Coatings
2.2.2. Oxidation Behavior and Mechanism of Plasma-Spraying Coatings
It should be noted that except for Mo2BC coating, the mass of the other coatings increases compared with that before oxidation. This is mainly due to the strong affinity force between C and oxygen. During oxidation, the volatilization rate of CO is greater than the formation rate of B2O3, resulting in the reduction of the overall quality of the coating.2.3. Coatings Prepared by Chemical Vapor Deposition (CVD) Technology
2.3.1. Microstructure and Growth Mechanism of CVD Coatings
| Substrate | Composition of Gas Mixture | Process Conditions | Composition and Thickness of Coatings (µm) | Bond Strength (MPa) | Hardness (GPa) | Surface Grain Size (μm) | Refs. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas Flow Rate (ml·min | −1 | ) | Deposition Temperature (°C) | Deposition Time (h) | Outerlayer | Interface Layer | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mo | SiCl | 4 | , H | 2 | SiO | 2 | (3.00)SiCl | 4 | : 50.00 H | 2 | : 100.00 | MoSi620.00 | 2 | (5.00)3.00 | SiO | 1000 °C, 3.00 h | - | 2 | (3.00) | SiO | 2 | , MoOMoSi | 2 | (5.00) | - | 3 | - | 15.00 | MoSi | 2 | -Mo | 5 | Si | 3[71] | [32] | ||||||||||||||||||||||||||||||
| 12.00 | [ | 71 | ] | [ | 32 | ] | NH | 3 | , SiCl | 4, | H | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| MoSi | 2 | -Si | 3 | N | 4 | (72.00)NH | 3 | : 100.00 H | 2 | : 990.00 SiCl | 4 | : 10.00 | 1100.00 | Mo | 2 | N (5.00)NH | 3 | : 2.00 SiCl | 4 | : 5.00 | MoSi | 2 | , Si | 3 | N | 4 | (72.00) | 500 °C, 1492.00 h | 1.00 h cycles | Si | 2 | ON | 2 | Mo | 2 | N (5.00) | - | , SiO | 2 | , MoO | 3 | Mo | 4 | O | 11 | , Mo | 9 | O | 26 | , (3.00) | MoSi | 2 | -Si | 3 | N | 4 | (100.00)- | 3.00 × 10 | −1 | 5.00 × 10 | −1 | [72] | [33] | [72] | [33] |
| BCl | 3 | , TiCl | 4 | , H | 2 | BCl | 3 | : 195.00 TiCl | 4 | : 130.00 H | 2 | : 635.00 | 1000.00 | 2.00 | TiB | 2 | (13.00) | ||||||||||||||||||||||||||||||||||||||||||||||||
| TiB | - | 7.00 | 28.00 | 2.00 | 2 | (13.00) | - | 900 °C, 6.00 h | - | TiO | 2 | , B | 2 | O | 3 | - | 8.00 × 10 | −2 | [73] | [ | [73] | [34] | |||||||||||||||||||||||||||||||||||||||||||
| 34 | ] | WCl | 2 | , H | 2 | - | 1800.00 | 2.00 | W (160.00) | - | - | - | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| TiB | 2 | (13.00) | - | 450 °C, 5.00 h | - | TiO | 2 | , B | 2 | O | 3 | 20.00 | - | 3.00 × 10 | −2 | [78] | [37] | [74] | [35] | ||||||||||||||||||||||||||||||||||||||||||||||
| CH | 4 | , SiCl | 4 | , H | 2 | CH | 4 | , H | 2 | :200.00 SiCl | 4 | : 10.00 H | 2 | : 990.00 | 1200.00, 1100.00 | CH | 4 | : 65.00 SiCl | 4 | : 10.00 | SiC, MoSi | 2 | (60.00) | MO | 2 | C (25.00) | - | - | 3.00 × 10 | −1 | [75] | [36] | |||||||||||||||||||||||||||||||||
2.3.2. Oxidation Behavior and Mechanism of CVD Coatings
Table 72 shows the microstructure evolution and mass gain of CVD coatings before and after oxidation under different conditions. Obviously, researchers mainly reported the oxidation of the coating at low temperature (500 °C to 1000 °C), and the oxidized coatings mainly consist of an oxide layer, intermediate layer and interface layer [71,72,73,74,75,78][32][33][34][35][36][37].| Substrate | Composition and Thickness of Coatings (µm) | Exposure | Comments | Composition and Thickness of Oxidized Coatings (µm) | Mass Gain (mg·cm | −2 | ) | Refs. | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outer Layer | Interface Layer | Oxide Layer | Intermediate Layer | ||||||||||||||||||||||||
| Mo | |||||||||||||||||||||||||||
| W (160.00) | |||||||||||||||||||||||||||
| W/Mo (2.00) | - | - | - | - | - | [ | 74] | [35] | |||||||||||||||||||
| MoSi | 2 | -SiC (60.00) | MO | 2 | C (25.00) | 500 °C, 1492.00 h | 1.00 h cycles | SiO | 2 | , MoO | 3 | Mo | 4 | O | 11 | , Mo | 9 | O | 26 | (8.00) | MoSi | 2 | -SiC (80.00) | 1.00 × 10 | −2 | [75] | [36] |
3. Conclusions
References
- Xu, J.J.; Yang, T.T.; Yang, Y.; Qian, Y.H.; Li, M.S.; Yin, X.H. Ultra-high temperature oxidation behavior of micro-laminated ZrC/MoSi2 coating on C/C composite. Corros. Sci. 2018, 132, 161–169.
- Khlyustova, A.; Sirotkin, N.; Titov, V.; Agafonov, A. Comparison of two types of plasma in contact with water during the formation of molybdenum oxide. Curr. Appl. Phys. 2020, 20, 1396–1403.
- Kong, H.; Kwon, H.S.; Kim, H.; Jeen, G.S.; Lee, J.; Lee, J.; Heo, Y.S.; Cho, J.; Jeen, H. Reductive-annealing-induced changes in Mo valence states on the surfaces of MoO3 single crystals and their high temperature transport. Curr. Appl. Phys. 2019, 19, 1379–1382.
- Bahamonde, J.P.; Wu, C.Z.; Louie, S.M.; Bao, J.M.; Rodrigues, D.F. Oxidation state of Mo affects dissolution and visible-light photocatalytic activity of MoO3 nanostructures. J. Catal. 2020, 381, 508–519.
- Smolik, G.R.; Petti, D.A.; Schuetz, S.T. Oxidation and volatilization of TZM alloy in air. J. Nucl. Mater. 2000, 283-287, 1458–1462.
- Majumdar, S.; Kapoor, R.; Raveendra, S.; Sinha, H.; Samajdar, I.; Bhargava, P.; Chakravartty, J.K.; Sharma, I.G.; Suri, A.K. A study of hot deformation behavior and microstructural characterization of Mo–TZM alloy. J. Nucl. Mater. 2009, 385, 545–551.
- Amakawa, K.; Wang, Y.Q.; Kröhnert, J.; Schlögl, R.; Trunschke, A. Acid sites on silica-supported molybdenum oxides probed by ammonia adsorption: Experiment and theory. Mol. Catal. 2019, 478, 110–580.
- Mannheim, R.L.; Garin, J.L. Structural identification of phases in Mo–Re alloys within the range from 5 to 95% Re. J. Mater. Process. Technol. 2003, 143-144, 533–538.
- Cui, K.K.; Zhang, Y.Y.; Fu, T.; Hussain, S.; Algarni, T.S.; Wang, J.; Zhang, X.; Ali, S. Effects of Cr2O3 Content on Microstructure and Mechanical Properties of Al2O3 Matrix Composites. Coatings 2021, 11, 234.
- Cui, Y.C.; Derby, B.; Li, N.; Misra, A. Fracture resistance of hierarchical Cu–Mo nanocomposite thin films. Mater. Sci. Eng. A 2021, 799, 139–891.
- Pan, Y.; Zhang, J. Influence of noble metals on the electronic and optical properties of the monoclinic ZrO2: A first-principles study. Vacuum 2021, 187, 110112.
- Li, J.C.; Wei, L.L.; He, J.; Chen, H. The role of Re in improving the oxidation-resistance of a Re modified PtAl coating on Mo-rich single crystal superalloy. J. Mater. Sci. Technol. 2020, 58, 63–72.
- Paul, B.; Kishor, J.; Majumdar, S.; Kain, V. Studies on growth mechanism of intermediate layer of (Mo,W)5Si3 and interdiffusion in the (Mo,W)-(Mo,W)Si2 system prepared by pack cementation coating. Surf. Interfaces 2020, 18, 100–458.
- Pan, Y. The structural, mechanical and thermodynamic properties of the orthorhombic TMAl (TM=Ti, Y, Zr and Hf) aluminides from first-principles calculations. Vacuum 2020, 181, 109742.
- Lu, Y.; Watanabe, M.; Miyata, R.; Nakamura, J.; Yamada, J.; Kato, H.; Yoshimi, K. Microstructures and mechanical properties of TiC-particulate-reinforced Ti–Mo–Al intermetallic matrix composites. Mater. Sci. Eng. A 2020, 790, 139–523.
- Cui, K.K.; Fu, T.; Zhang, Y.Y.; Wang, J.; Mao, H.B.; Tan, T.B. Microstructure and mechanical properties of CaAl12O19 reinforced Al2O3-Cr2O3 composites. J. Eur. Ceram. Soc. 2021, 41, 7935–7945.
- Niu, F.X.; Wang, Y.X.; Wang, Y.Y.; Ma, L.R.; Liu, J.J.; Wang, C.G. A crack-free SiC nanowire-toughened Si-Mo-W-C coating prepared on graphite materials for enhancing the oxidation resistance. Surf. Coat. Technol. 2018, 344, 52–57.
- Zhang, Y.Y.; Fu, T.; Cui, K.K.; Shen, F.Q.; Wang, J.; Yu, L.H.; Mao, H.B. Evolution of surface morphology, roughness and texture of tungsten disilicide coatings on tungsten substrate. Vacuum 2021, 191, 110297.
- Jiang, C.; Mariani, R.D.; Adkins, C.A. Ab initio investigation and thermodynamic modeling of the Mo–Ti–Zr system. Materialia 2020, 10, 100–701.
- Zhao, M.; Xu, B.Y.; Shao, Y.M.; Zhu, Y.; Wu, J.; Wu, S.S.; Yan, Y.W. Microstructure and oxidation mechanism of multiphase Mo–Ti–Si–B alloys at 800 °C. Corros. Sci. 2021, 187, 109518.
- Zheng, X.Q.; Liu, Y. Slurry erosion–corrosion wear behavior in SiC-containing NaOH solutions of Mo2NiB2 cermets prepared by reactive sintering. Int. J. Refract. Met. Hard Mater. 2019, 78, 193–200.
- Gao, J.S.; Liu, Z.M.; Yan, Z.Q.; He, Y. A novel slurry blending method for a uniform dispersion of carbon nanotubes in natural rubber composites. Results Phys. 2019, 15, 102–720.
- Li, W.; Fan, J.L.; Fan, Y.; Xiao, L.R.; Cheng, H.C. MoSi2/(Mo, Ti)Si2 dual-phase composite coating for oxidation protection of molybdenum alloy. J. Alloys Compd. 2018, 740, 711–718.
- Cai, Z.Y.; Liu, S.N.; Xiao, L.R.; Fang, Z.; Li, W.; Zhang, B. Oxidation behavior and microstructural evolution of a slurry sintered Si-Mo coating on Mo alloy at 1650 °C. Surf. Coat. Technol. 2017, 324, 182–189.
- Chakraborty, S.P. Development of Protective Coating of MoSi2 over TZM Alloy Substrate by Slurry Coating Technique. Mater. Today Proc. 2016, 3, 3071–3076.
- Cai, Z.Y.; Wu, Y.H.; Liu, H.Y.; Tian, G.Y.; Pu, R.; Piao, S.M.; Tang, X.Y.; Liu, S.N.; Zhao, X.J.; Xiao, L.R. Formation and oxidation resistance of a new YSZ modified silicide coating on Mo-based alloy. Mater. Des. 2018, 155, 463–474.
- Vaunois, J.R.; Poulain, M.; Kanouté, P.; Chaboche, J.L. Development of bending tests for near shear mode interfacial toughness measurement of EB-PVD thermal barrier coatings. Eng. Fract. Mech. 2017, 171, 110–134.
- Gupta, M.; Li, X.H.; Markocsan, N.; Kjellman, B. Design of high lifetime suspension plasma sprayed thermal barrier coatings. J. Eur. Ceram. Soc. 2020, 40, 768–779.
- Gorr, S.M.B.; Christ, H.J.; Schliephake, D.; Heilmaier, M. Oxidation mechanisms of lanthanum-alloyed Mo–Si–B. Corros. Sci. 2014, 88, 360–371.
- Zhang, Y.; Pint, B.A.; Cooley, K.M.; Haynes, J.A. Effect of nitrogen on the formation and oxidation behavior of iron aluminide coatings. Surf. Coatings Technol. 2005, 200, 1231–1235.
- Pochet, L.F.; Howard, P.; Safaie, S. Practical aspects of deposition of CVD SiC and boron silicon carbide onto high temperature composites. Surf. Coat. Technol. 1996, 86-87, 135–141.
- Nyutu, E.K.; Kmetz, M.A.; Suib, S.L. Formation of MoSi2–SiO2 coatings on molybdenum substrates by CVD/MOCVD. Surf. Coat. Technol. 2006, 200, 3980–3986.
- Yoon, J.K.; Kim, G.H.; Han, J.H.; Shon, I.J.; Doh, J.M.; Hong, K.T. Low-temperature cyclic oxidation behavior of MoSi2/Si3N4 nanocomposite coating formed on Mo substrate at 773 K. Surf. Coat. Technol. 2005, 200, 2537–2546.
- Huang, X.X.; Sun, S.C.; Lu, S.D.; Li, K.H.; Tu, G.F.; Song, J.X. Synthesis and characterization of oxidation-resistant TiB2 coating on molybdenum substrate by chemical vapor deposition. Mater. Lett. 2018, 228, 53–56.
- Du, J.H.; Li, Z.X.; Liu, G.J.; Zhou, H.; Huang, C.L. Surface characterization of CVD tungsten coating on molybdenum substrate. Surf. Coat. Technol. 2005, 198, 169–172.
- Yoon, J.K.; Lee, K.H.; Kim, G.H.; Han, J.H.; Doh, J.M.; Hong, K.T. Low-Temperature Cyclic Oxidation Behavior of MoSi2/SiC Nanocomposite Coating Formed on Mo Substrate. Mater. Trans. 2004, 45, 2435–2442.
- Huang, X.X.; Sun, S.C.; Tu, G.F. Investigation of mechanical properties and oxidation resistance of CVD TiB2 ceramic coating on molybdenum. J. Mater. Res. Technol. 2020, 9, 282–290.
