Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 1 by Junho Jeon.
The scientific community has increasingly focused on forming transformation products (TPs) from environmental organic pollutants. However, there is still a lot of discussion over how these TPs are generated and how harmful they are to living terrestrial or aquatic organisms. Potential transformation pathways, TP toxicity, and their mechanisms require more investigation.
- environmental contamination
- transformation products
- non-target screening
1. Introduction
Concerns about emerging pollutants (EPs) have increased in recent years due to their prevalence in the environment and the potential for deleterious effects on the environment [1][2][3][4]. Effluent discharges from industrial wastewater treatment plants (WWTPs), municipal, hospital, sewer overflow/sewer leakage, and surface runoff from agricultural or urban areas can all introduce EPs to the aquatic environment [5][6][7][8][9]. A special focus has been paid to WWTPs due to the relatively frequent release and high contribution of EPs into the environment. Raw influent and treated effluent commonly include EPs at concentrations ranging from ng/L to mg/L [10][11][12][13][14]. The socioeconomic composition of the population feeding into WWTPs impacts the concentrations and types of EPs in wastewaters. EPs in the water environment have often been accumulated in aquatic organisms and lead to alterations that endanger the sustainability of aquatic ecosystems [15][16].
In vitro and in vivo metabolic processes may transform organic pollutants into highly reactive metabolites. The principal biotransformation routes accompany reduction, oxidation, and/or hydrolysis during the phase I reaction. In contrast, the phase II reaction mainly features conjugation reactions. Phase II reactions are biosynthetic, as active enzymes connect the metabolite produced by phase I responses to an endogenous polar molecule, resulting in a conjugate. Many endogenous compounds with high polarity (e.g., sugar, amino acids, sulphates, etc.) are used in conjugation, and the resultant conjugates are mostly ionized and highly water soluble. Furthermore, specialized active transport systems identify the moieties employed for conjugation, assisting translocation across plasma membranes and increasing the excretion rate [107,108][17][18]. The endoplasmic reticulum, lipoprotein membranes stretching from mitochondria and nucleus to the plasma membranes of cells, are the primary sources of phase I enzymes in cells. As lipophilic substances preferentially diffuse into lipid membranes the presence of phase I enzymes in lipid membranes has crucial implications for biotransformation [109][19].
Phase I reactions are more typically related to the production of reactive and more hazardous metabolites; yet, phase II processes, as well as combinations of phase II and phase I processes, may be considered an intoxication procedure [110,111][20][21]. However, there is evidence that metabolites of pollutants such as tetrabromobisphenol-A, trenbolone, triclosan, and bisphenol A retain the bioactive moieties and preserve inherent toxicity comparable to the parent compound [112,113,114][22][23][24]. Methylation in biological systems can produce hydrophobic and bioaccumulative metabolites, often observed in fungus, plants, and bacteria [115][25]. Compound biotransformation studies are vital to understand the reactivity and toxicity of organisms. Bioaccumulation and toxicity of organic pollutants are heavily influenced by biotransformation, while this process is still poorly understood for emerging contaminants [116][26]. There have been limited investigations for TPs of EPs in specific organisms, as follows.
2. Algae
Cymbella sp. were studied for their ability to biotransform triclosan. The results demonstrated that triclosan and its potential hazardous metabolites had a high toxic impact on Cymbella sp., with 72 h EC50 of 324.9 mg/L. In diatom cells, 11 metabolites were found and with potential degradation pathways. The transformative reactions of triclosan in Cymbella sp. included methylation, hydroxylation, amino acids conjunction, dichlorination, and glucuronidation, which resulted in biologically active products (e.g., methyl triclosan) and conjugation products (e.g., or oxaloacetic acid conjugated or triclosan glucuronide) [117][27].
3. Freshwater Crustaceans
Biotransformation pathways in freshwater crustaceans have been little understood, except in a study using Gammarus pulex (G. pulex) and Daphnia magna (D. magna). For 24 h, G. pulex and D. magna were exposed to a modest dose of biocides and pharmaceuticals and sacrificed to identify their metabolites. Each species produced 25 and 11 metabolites, respectively, for terbutryn, irgarol, venlafaxine, and tramadol, mainly via oxidation and conjugation reactions. Affinity in the synthesis of metabolites, such as oxidation and demethylation products, were found for venlafaxine and tramadol, which have an identical backbone structure. Tramadol and venlafaxine were oxidized at the amine or cyclic C-H bond, while irgarol and terbutryn were oxidized at the terminal methyl group (MTE258B, MIR270B, MIR270A, MTE258A, and MIR286) (Figure 4) [118][28]. In Gammarus pulex (G. pulex) and Hyalella azteca (H. azteca), Fu et al. (2020) found a substantial pathway for diclofenac metabolism [119][29]. The LC–HRMS/MS data collected from the test species were used to identify metabolites utilizing NTS procedures. As a result, 281 metabolites were identified based on the isotopic signature of chlorine (Cl). H. azteca and G. pulex had nine distinct diclofenac metabolites.
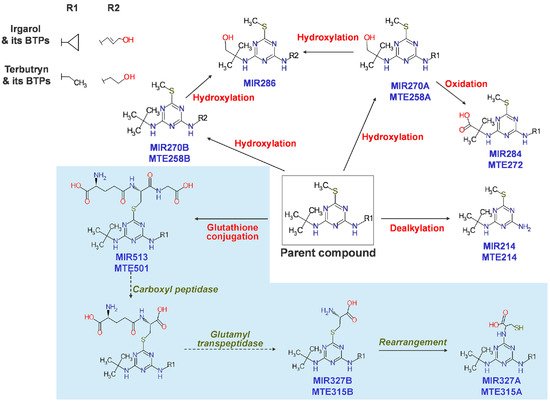
Figure 4. Proposed biotransformation pathways of irgarol and terbutryn in freshwater crustaceans. Note that R2 is the hydroxylated moiety of R1. The sky-blue shaded area indicates a pathway including glutathione conjugation followed by subsequent reactions to form cysteine conjugates, reported for the first time in the test organisms. (Reprinted from [118][28], Copyright 2013, ACS).
4. Fish
Metabolites of diclofenac were also identified in vertebrates. Oncorhynchus mykiss, have produced hydroxylate and conjugate of diclofenac with glucuronic acid, glutathione, and sulfate. Other EPs, including pharmaceuticals (propranolol, carbamazepine) and insecticides (diazinon, azoxystrobin, and fipronil), were found in the S9 extract of trout liver [120][30]. It was revealed that each of the five parents had ten distinct metabolites. The primary metabolic mechanisms were oxidation, dealkylation, S-oxidation, and epoxidation. The formation of metabolites for fipronil and diazinon was enhanced as increasing carbamazepine concentration in the binary exposure, whereas the transformation kinetic for propranolol and azoxystrobin was decreased. Toxic diazoxon and less toxic pyrimidinol, among significant diazinon metabolites, were promptly formed by S9 after the binary exposure with carbamazepine.
5. Earthworm
High-production-volume surfactants, also known as polyfluoroalkyl phosphate esters (PAPs), are employed in the packaging industry and food contact paper. PAPs can transform into perfluoroalkyl carboxylic acids, which are highly bioaccumulative and persistent in the environment, although their fate remains unknown in terrestrial species. To investigate biotransformation, Zhu et al. (2021) subjected M. guillelmi to soil contaminated with 6:2 fluorotelomer phosphate diester (6:2 diPAP). According to in vitro desorption tests [121][31], 6:2 diPAP desorbed from soil was considerably accumulated in gut digesting fluid. Phase I products included perfluoropentyl propanoic acid, perfluorohexanoic acid, 2-perfluorohexyl ethanoic acid, perfluoropentanoic acid, and perfluoroheptanoic acid, all of which confirmed that β and α oxidation occurred in earthworms. As a phase II product, 6:2 fluorotelomer alcohol–sulfate conjugate was found at unusually high quantities in earthworms for the first time, which may be the principal mechanism by which earthworms remove 6:2 diPAP.
6. Human Cell Lines
Using human skin subcellular fractions, the in vitro metabolism of 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and a mixture of Bis(2-ethylhexyl) tetrabromphthalate (BEH-TEBP), EH-TBB, and triphenyl phosphate was evaluated for the first time. Analysis of EH-TBB and THP utilizing UPLC-Q-Exactive Orbitrap identified the two primary metabolites, tetrabromobenzoic acid (TBBA) and diphenylphosphate (DPhP). It was assumed that CYP450 enzymes were responsible for the dermal biotransformation of TPhP and EH-TBB, but no stable metabolites were found for BEH-TEBP [122][32].
References
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil—A critical review. Sci. Total Environ. 2015, 538, 750–767.
- Ferrari, B.; Paxéus, N.; Giudice, R.L.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370.
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155.
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25.
- Wolf, L.; Zwiener, C.; Zemann, M. Tracking artificial sweeteners and pharmaceuticals introduced into urban groundwater by leaking sewer networks. Sci. Total Environ. 2012, 430, 8–19.
- Yi, X.; Tran, N.H.; Yin, T.; He, Y.; Gin, K.Y.H. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017, 121, 46–60.
- Launay, M.A.; Dittmer, U.; Steinmetz, H. Organic micropollutants discharged by combined sewer overflows–characterisation of pollutant sources and stormwater-related processes. Water Res. 2016, 104, 82–92.
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 2009, 38, 1086–1108.
- Sidhu, J.P.; Ahmed, W.; Gernjak, W.; Aryal, R.; McCarthy, D.; Palmer, A.; Kolotelo, P.; Toze, S. Sewage pollution in urban stormwater runoff as evident from the widespread presence of multiple microbial and chemical source tracking markers. Sci. Total Environ. 2013, 463, 488–496.
- Behera, S.K.; Kim, H.W.; Oh, J.E.; Park, H.S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360.
- Tran, N.H.; Hu, J.; Ong, S.L. Simultaneous determination of PPCPs, EDCs, and artificial sweeteners in environmental water samples using a single-step SPE coupled with HPLC–MS/MS and isotope dilution. Talanta 2013, 113, 82–92.
- Tran, N.H.; Hu, J.; Li, J.; Ong, S.L. Suitability of artificial sweeteners as indicators of raw wastewater contamination in surface water and groundwater. Water Res. 2014, 48, 443–456.
- Tran, N.H.; Li, J.; Hu, J.; Ong, S.L. Occurrence and suitability of pharmaceuticals and personal care products as molecular markers for raw wastewater contamination in surface water and groundwater. Environ. Sci. Pollut. Res. 2014, 21, 4727–4740.
- Tran, N.H.; Urase, T.; Ta, T.T. A preliminary study on the occurrence of pharmaceutically active compounds in hospital wastewater and surface water in Hanoi, Vietnam. CLEAN–Soil Air Water 2014, 42, 267–275.
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Potential ecological and human health risks associated with the presence of pharmaceutically active compounds in the aquatic environment. Crit. Rev. Toxicol. 2004, 34, 335–350.
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303.
- Dekant, W. The role of biotransformation and bioactivation in toxicity. Mol. Clin. Environ. Toxicol. 2009, 99, 57–86.
- Adams, J.P.; Collis, A.J.; Henderson, R.K.; Sutton, P.W. Biotransformations in small-molecule pharmaceutical development. Pract. Methods Biocatal. Biotransformations 2010, 1–82.
- Sinclair, C.J.; Boxall, A.B. Assessing the ecotoxicity of pesticide transformation products. Environ. Sci. Technol. 2003, 37, 4617–4625.
- Brandsma, S.H.; Sellström, U.; de Wit, C.A.; de Boer, J.; Leonards, P.E. Dust measurement of two organophosphorus flame retardants, resorcinol bis (diphenylphosphate) (RBDPP) and bisphenol A bis (diphenylphosphate) (BPA-BDPP), used as alternatives for BDE-209. Environ. Sci. Technol. 2013, 47, 14434–14441.
- deBethizy, J.D.; Hayes, J.R. Metabolism: A determinant of toxicity. Princ. Methods Toxicol. 1994, 59–100.
- Arand, M.; Cronin, A.; Oesch, F.; Mowbray, S.L.; Alwyn Jones, T. The telltale structures of epoxide hydrolases. Drug Metab. Rev. 2003, 35, 365–383.
- Arand, M.; Cronin, A.; Adamska, M.; Oesch, F. Epoxide hydrolases: Structure, function, mechanism, and assay. Methods Enzymol. 2005, 400, 569–588.
- Tukey, R.H.; Strassburg, C.P. Human UDP-glucuronosyltransferases: Metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581–616.
- Duffel, M.W.; Marshall, A.D.; McPhie, P.; Sharma, V.; Jakoby, W.B. Enzymatic aspects of the phenol (aryl) sulfotransferases. Drug Metab. Rev. 2001, 33, 369–395.
- Vogel, C. Prostaglandin H synthases and their importance in chemical toxicity. Curr. Drug Metab. 2000, 1, 391–404.
- Ding, T.; Lin, K.; Bao, L.; Yang, M.; Li, J.; Yang, B.; Gan, J. Biouptake, toxicity and biotransformation of triclosan in diatom Cymbella sp. and the influence of humic acid. Environ. Sci. Pollut. 2018, 234, 231–242.
- Jeon, J.; Kurth, D.; Hollender, J. Biotransformation pathways of biocides and pharmaceuticals in freshwater crustaceans based on structure elucidation of metabolites using high resolution mass spectrometry. Chem. Res. Toxicol. 2013, 26, 313–324.
- Fu, Q.; Fedrizzi, D.; Kosfeld, V.; Schlechtriem, C.; Ganz, V.; Derrer, S.; Rentsch, D.; Hollender, J. Biotransformation changes bioaccumulation and toxicity of diclofenac in aquatic organisms. Environ. Sci. Technol. 2020, 54, 4400–4408.
- Jeon, J.; Hollender, J. In vitro biotransformation of pharmaceuticals and pesticides by trout liver S9 in the presence and absence of carbamazepine. Ecotoxicol. Environ. Saf. 2019, 183, 109513.
- Zhu, Y.; Jia, Y.; Liu, M.; Yang, L.; Yi, S.; Feng, X.; Zhu, L. Mechanisms for tissue-specific accumulation and phase I/II transformation of 6: 2 fluorotelomer phosphate diester in earthworm (M. guillelmi). Environ. Int. 2021, 151, 106451.
- Abdallah, M.A.E.; Nguyen, K.H.; Moehring, T.; Harrad, S. First insight into human extrahepatic metabolism of flame retardants: Biotransformation of EH-TBB and Firemaster-550 components by human skin subcellular fractions. Chemosphere 2019, 227, 1–8.
More
