Surgical technique and technology frequently coevolve. The brief history of blood vessel anastomosis is full of famous names. While the techniques pioneered by these surgeons have been well described, the technology that facilitated their advancements and their inventors deserve recognition. The mass production of laboratory microscopes in the mid-1800s allowed for an explosion of interest in tissue histology. This improved understanding of vascular physiology and thrombosis laid the groundwork for Carrel and Guthrie to report some of the first successful vascular anastomoses. In 1916, McLean discovered heparin. Twenty-four years later, Gordon Murray found that it could prevent thrombosis when performing end-to-end anastomosis. These discoveries paved the way for the first-in-human kidney transplantations. Otolaryngologists Nylen and Holmgren were the first to bring the laboratory microscope into the operating room, but Jacobson was the first to apply these techniques to microvascular anastomosis. His first successful attempt in 1960 and the subsequent development of microsurgical tools allowed for an explosion of interest in microsurgery, and several decades of innovation followed.
- surgical history
- anastomosis
- innovation
- surgical technology
- microsurgery
- vascular surgery
1. Introduction
2. Early Vascular Repair (before 1900)
3. The Birth of Microscopy and Early Techniques (1850s to 1940s)
3.1. First Successful Blood Vessel Anastomoses
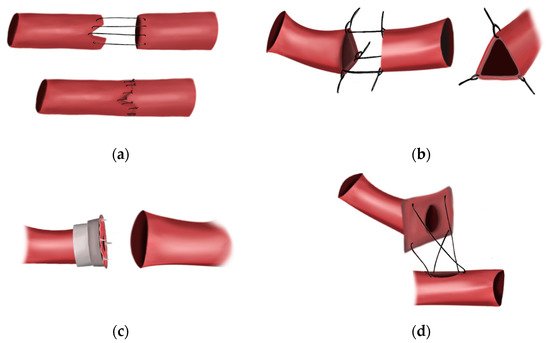
3.2. First Interposition Vein Grafts
3.3. Sutureless Anastomosis
3.4. Discovery of Heparin

3.5. First Clinical Applications of Vascular Anastomosis
4. The Birth of Microsurgery
4.1. First Microvascular Surgery
4.2. Development of Microsurgical Tools
4.2.1. Needle Holders
4.2.2. Suture Material
4.2.3. Vascular Clamps
4.2.4. Electrocautery
4.2.5. Venous Coupler
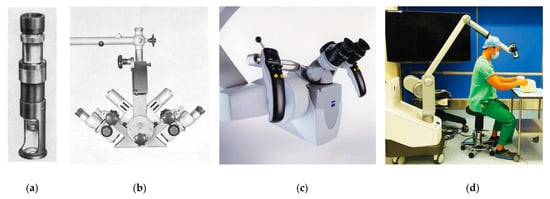
4.2.6. Double-Binocular Microscope
| Year | Achievement | Innovator |
|---|---|---|
| 1964 | Zeiss Diploscope | Littman [6] |
| 1964 | First arm replantation | Malt and McKhann [50] |
| 1965 | First total thumb replantation | Komatsu and Tamai [45] |
| 1965 | Experimental thumb replantation | Buncke [51] |
| 1966 | First toe-to-thumb transplantation | Buncke [52] |
| 1966 | First total ear replantation | Buncke [53] |
| 1972 | Omentum transfer for scalp reconstruction | Buncke and McLean [54] |
| 1973 | First free skin flap | Daniel and Taylor [55] |
| 1974 | First pectoralis major transfer | Harii [56] |
| 1975 | First free fibular flap | Taylor [57] |
| 1975 | First free dorsalis pedis flap | McGraw [58] |
| 1977 | First penile and scrotal replantation | Tamai [59] |
| 1982 | First free scapular flap | Gilbert [60] |
| 1984 | First free peroneal flap | Yoshimura [61] |
4.3. Development of Supermicrosurgery
5. New Directions and Outlook
5.1. Exoscopes
During the present era, operating microscopes have become increasingly sophisticated through the additions of cameras for recording procedures and pedals for adjusting magnification, all while offering high-quality images of the field [6]. However, operating microscopes still have room for improvement, as they can be bulky and inflexible. In contrast, loupes offer a lightweight and maneuverable alternative; however, they cannot change magnification or focal length [6]. Future surgical loupes might use cameras integrated into the frame, allowing for automated digital magnification of the surgical field. However, there would be significant engineering challenges for such development. Importantly, technology would have to make advanced assumptions to detect and focus objects of interest in the surgeon’s operative field. Although loupe-only “macro-level microsurgery” is possible with vessels above 1.5 mm in diameter [71], microscopes are still considered essential for many supermicrosurgery applications [71]. Extracorporeal telescopes, or exoscopes, employ a number of high-definition digital cameras around the operating room to provide surgeons with a magnified video display of the operative field [72]. This new technology offers an alternative to surgical loupes and the operative microscope. At MD Anderson Cancer Center in Houston, TX, USA, no significant differences in operative times and surgical complications were found between exoscopes and operative microscopes. However, surgeons reported less physical discomfort while using the exoscope, a factor that could make it a more attractive option to microsurgeons in the future [72].
5.2. Robot-Assisted Microsurgery
Robot-assisted procedures have appeared on the horizon of supermicrosurgery to help overcome the limitations of performing such procedures that challenge the natural boundaries of manual dexterity. In 2020, van Mulken et al. conducted a randomized trial to pilot robotic supermicrosurgery to treat breast-cancer-related lymphedema in 22 females [73]. This technology is not foreign to surgery; the da Vinci (Intuitive Surgical Inc., Sunnyvale, CA, USA) is used to perform minimally invasive procedures in a variety of subspecialities, mainly laparoscopic and thoracoscopic procedures [73]. However, in the field of microsurgery, the da Vinci poses some critical limitations, namely its poorer resolution at high levels of magnification [73]. These limitations led to the development of the MUSA (MicroSure, Eindhoven, The Netherlands), the world’s first robot dedicated for use in supermicrosurgery [73]. With the ability to dampen natural fine tremors, the MUSA can manipulate surgical instruments with ease and accuracy, as demonstrated in preclinical trials [74,75]. The study conducted in 2020 by van Mulken et al. was successful in showcasing the feasibility of the use of MUSA in accomplishing lymphovenous anastomosis, with comparable results at one and three months post-operatively to the manual procedure [73].
5.3. Sutureless Anastomotic Devices
Other avenues for improvement of vascular anastomosis could include forgoing sutured anastomosis entirely. For as long as surgeons have been sewing vessels together, innovators have tried to avoid sutures entirely [17]. Sutured anastomosis is time-consuming, and outcomes are highly dependent on the skill of the surgeon. There is a need to develop adhesives or devices that will remain in place permanently and securely without risk of thrombosis. Today, vascular shunts are utilized in the military to restore blood flow while wounded soldiers await definitive reconstruction outside of the combat theater [76,77]. However, these devices are only designed to remain in place temporarily. In 2016, Jose and colleagues [78] published preliminary laboratory results on a prototype anastomotic device made of a deposited in 40 µm monolayers. They showed that their device is potentially resorbable in vivo, potentially allowing for long-term placement, and could be secured in under a minute [78].
5.4. Tissue Adhesion
In 1982, Wintermantel described the creation of vascular anastomoses without sutures or permanent intralumenal implants [79]. He designed wire loops that would both approximate vessel ends and conduct an applied electrical current. The resulting heat would serve to fuse the vessels together. This technique showed remarkable success in Wintermantel’s rat model of carotid artery anastomosis, with 90% of anastomoses remaining patent after 30 days. The application of a muscle strip to these anastomoses with fibrin glue resulted in 100% patency after 6 months. The use of tissue adhesives alone has been a focus of ongoing research [80,81]. Adhesives such as cyanoacrylate are readily available in the clinical setting but, until recently, have not been successfully employed in vascular anastomosis, mainly due to challenges in maintaining luminal patency during their application. Recently, researchers have paired these adhesives with surgical stents made of poloxamers, water-soluble structures in current clinical use for drug delivery [82,83]. Poloxamers demonstrate thermo-reversibility between liquid and semisolid gel, allowing for them to temporarily hold vessels patent during application of tissue adhesives before returning to liquid state and allowing for restoration of blood flow. These stents have shown promise, resulting in increased patency and a wider lumen than conventional sutured anastomosis when paired with tissue adhesives [82,83,84]. Laser-assisted vessel-welding technologies, specifically those employing photothermal modalities, offer another potential alternative to traditional sutured anastomoses. These instruments produce heat by delivering light to endogenous chromophores on the vessel surface. This heat denatures and cross-links collagen molecules, allowing for adhesion without the use of tissue adhesives [85]. While this method has many potential advantages, such as minimizing foreign-body reaction and liquid-tight sealing, further research is required for this method to be brought into the operating room. The current modalities require stay sutures to obtain welding strengths equivalent to sutured anastomoses. Furthermore, this method poses the risk of damaging vessels through thermal diffusion [85].
5. Conclusions
Today, microvascular and vascular anastomosis can be performed on vessels of practically every size with consistently excellent results. We frequently remember the names of the surgeons who first demonstrated that these surgeries were possible. However, many of these successes were the culmination of decades of technological innovation (Figure 4). With the mass production of the laboratory microscope in the 1800s, Murphy, Carrel and Guthrie were able to apply new understandings of vessel physiology to successfully achieve vessel anastomosis in the laboratory; by discovering heparin, Jay McLean allowed vascular anastomosis to be transitioned out of the laboratory and into the clinical setting; and after Carl Nylen bringing a laboratory microscope into the operating room in the 1920s, Jacobson was able apply this technology to vessel anastomosis. As we look to push the boundaries of vascular anastomosis further, we can expect additional technological advances to improve our ability to place blood vessels of all sizes together with efficiency, patency and improved clinical outcomes.
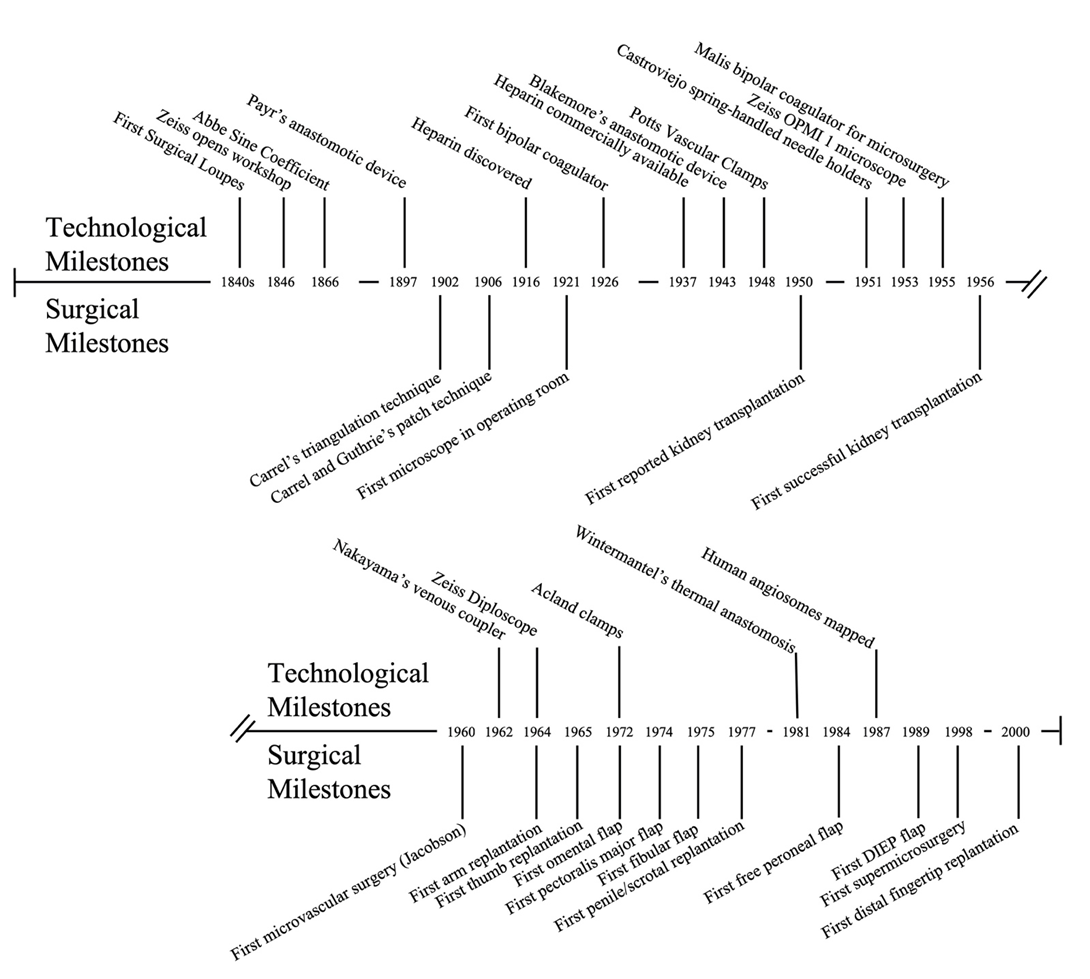
Figure 4. Technological and surgical milestones of blood vessel anastomosis.
