Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sujie Chang and Version 2 by Amina Yu.
Self-assembled nanocomposites and nanostructures are a class of molecules that spontaneously assemble directly through specific interactions or indirectly through their environment without any human intervention. The benefits of self-assembled nanocomposites or nanostructures have been reported several times for their prominent applications.
- nanocomposites
- nanostructures
- self-assembled
- photocatalyst
- energy materials
- thermoelectric materials
1. Self-Assembled Nanostructured Membranes for Photocatalysis
1.1. Self-Assembled Nanostructured Membrane Photocatalysts for Wastewater Treatment
Liu et al. prepared titanium dioxide films via a layer-by-layer self-assembly technique to immobilize TiO2 nanoparticles using polyurethane (PU) and increase the adsorption capacity of the photocatalyst [1][58]. In addition, the photocatalytic performance and reusability of the films were investigated by the decomposition of MB under UV irradiation, and it was shown that the catalytic efficiency of the prepared films was still as high as 94.56% after five cycles and they could be reused six times without affecting the photocatalytic activity. Synthetic membranes are therefore promising candidate materials for wastewater treatment applications.
However, single nano-TiO2 photocatalysts have low specific surface area, poor adsorption capacity for pollutants, and are easily agglomerated and difficult to recover, resulting in low catalytic efficiency for the photocatalytic degradation of low concentrations of organic matter. The researchers found that composite nano-TiO2 materials can significantly improve the defects of single nano-TiO2 materials which are prone to agglomeration. To overcome the defects of nano-TiO2 particles, research and application of composite materials are gaining increasing attention [2][3][4][21,22,23].
Zhang et al. successfully coated TiO2 nanofibers onto ceramic hollow fiber membranes using a simple dip-coating technique to form TiO2 nanofiber membranes with reticular morphologies (Figure 4a), and they evaluated the performances of the TiO2 nanofiber membranes in treating hyaluronic acid by monitoring the change in the total organic carbon values in water [5][59]. After six cycles, there was no significant loss of activity of the TiO2 nanofiber hollow membrane (Figure 4c). Therefore, the TiO2 nanofiber hollow membranes proposed possessed high stability during the removal of hyaluronic acid.
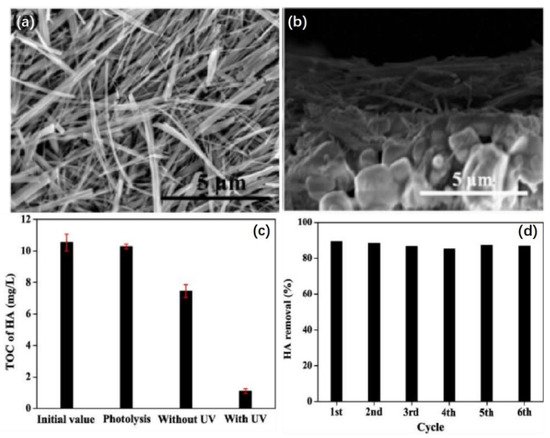
Figure 4. (a) Scanning electron microscopy (SEM) image of TiO2 nanofibers synthesized by the hydrothermal method. (b) SEM image of ceramic hollow membranes coated with TiO2 nanofibers. (c) Total organic carbon (TOC) removal using TiO2 nanofiber membranes. (d) Reusability of the TiO2 nanofiber membranes for hyaluronic acid removal. Copyright © 2015 Elsevier B.V.
Bai et al. successfully synthesized a novel multifunctional carbon nanotube/ZnO/TiO2 nanocomposite ultrafiltration membrane by hydrothermal synthesis and ultrasonic-assisted acid treatment [6][60]. Chang et al. obtained self-assembled nanoporous Ti with a smooth surface and many folds by a hydrothermal method using metallic titanium foam as the raw material [7](Figure 5a,b) [61]. A self-assembled layer of a strongly adherent 3D Na2Ti3O7 nanowire network was grown on the surfaces of Ti particles and channels after alkaline hydrothermal treatment in a NaOH solution (Figure 5c). The self-assembled TiO2 nanowire networks were uniform, with lengths of 2–3 μm (Figure 5d) [7][61]. The self-assembled nanowire network utilized the wastewater degradation device shown in Figure 5e for two different types of dyes, RhB (20 mg/L, Figure 5f) and MB (20 mg/L, Figure 5g), both of which showed good photocatalytic properties after UV irradiation for 60 min only, reflecting its good degradation effects. The above results illustrate the high performance of self-assembled nanoporous Ti as photoelectrolytic electrode materials.
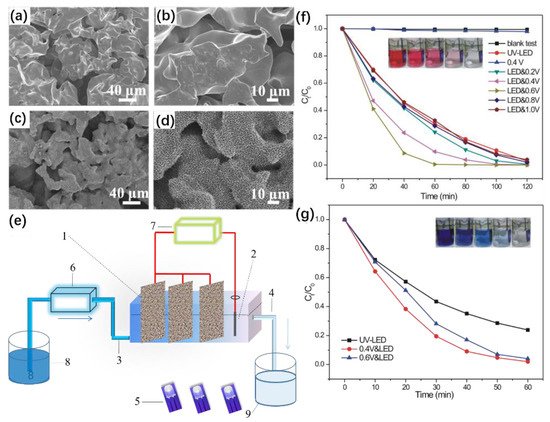
Figure 5. (a,b) SEM images of porous Ti. (c) Na2Ti3O7/porous Ti. (d) SEM images of the cross-section of TiO2/porous Ti with different magnifications. (e) Schematic diagram of the experimental reactor system: 1—TiO2/porous Ti, 2—Pt wire electrode, 3—water inlet, 4—water outlet, 5—UV-light-emitting diodes (LEDs), 6—peristaltic pump, 7—electrochemical workstation, 8—sewage pool, and 9—recovered water pool. (f) Ct/C0 vs. t and (g) MB degradation (20 mg/L) using TiO2/porous Ti under photoelectrocatalysis (PEC) conditions. Copyright © 2017 Elsevier B.V.
In addition, Fe3O4-based nanocomposites can be used as good Fenton-like catalysts for the degradation of organic pollutants in water [8][62]. The introduction of magnetic Fe3O4 nanoparticles provides another advantage for nanostructured composites, and the magnetic properties of the prepared composites facilitate fast and easy separation during catalyst recovery and reuse [9][63].
Wang et al. successfully prepared an Fe3O4/rGO/metal–organic framework (MOF) composite with a dispersed interlayer structure by a hydrothermal method (Figure 6a) and investigated the degradation performances of these composites on phenol [10][64]. They found that the degradation of phenol was mainly dependent on the pH, and the degradation efficiency of phenol reached 80% within 2 min at pH = 3. The degradation rate of phenol decreased sharply when the pH was further reduced [11][65]. The phenol was completely removed within 16 min for all pH conditions (Figure 6b). By exploring the role of the catalyst components in Fenton-like reactions (Figure 6c), they found that the excellent catalytic performances of the Fe3O4/rGO/MOF composites were mainly due to the synergistic effect of the porous MOF shell and the internal Fe3O4/rGO [12][66]. The reusability of Fe3O4/rGO/MOF was tested by recovering the catalyst at the end of the reaction and reusing it in the next run. As shown, the catalytic activity of Fe3O4/rGO/MOF was maintained at 96% after five reuses (Figure 6d).
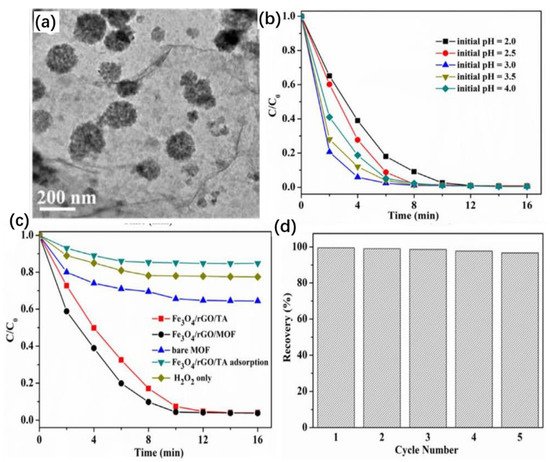
Figure 6. (a) TEM image of the as-prepared Fe3O4/reduced graphene oxide (rGO)/metal–organic framework (MOF). (b) Effect of initial pH on degradation of phenol. (c) Effect of catalyst dose on the degradation of phenol. (d) Reusability of Fe3O4/rGO/MOF. Copyright © 2019 Taiwan Institute of Chemical Engineers.
2. Self-Assembled Nanostructured Membrane Photocatalysts for Photocatalytic Hydrogen Production
Photocatalyst modification using doped noble metal nanoparticles is an effective method to improve the photocatalytic performance. Dal’Acqua et al. prepared a multilayer composite by combining gold (Au) and titanium dioxide (TiO2) nanoparticles (NPs) into self-assembled photocatalytic films (SAPFs) [13](Figure 7a) [70], forming a composite with larger specific surface area compared to those of conventional nanostructured catalysts. This facilitated the maximization of the photocatalytic activity. In this structured photocatalyst, hydrogen is produced in the polymer/(TiO2-Au) nanoparticle network and also in the body of the polymer/(TiO2-Au) NP assembly. Hydrogen is readily produced in large quantities under radiation, and the amount of hydrogen produced by the structured photocatalyst increased linearly with increasing UV irradiation time (Figure 7b). The SAPFs have great potential for renewable energy development due to their simple preparation process and excellent photocatalytic activity.
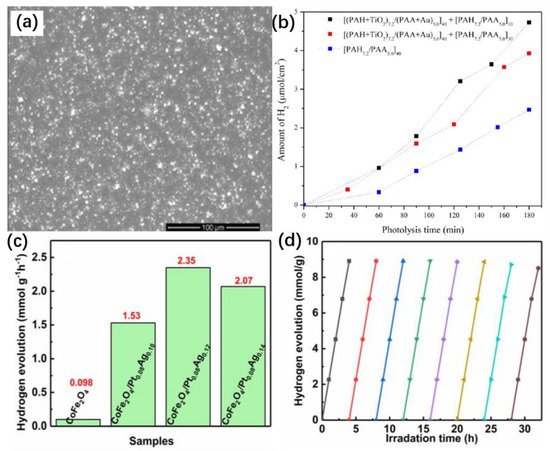
Figure 7. (a) SEM images of self-assembled photocatalytic films (SAPF) with 60 layers on a silicon substrate. (b) Hydrogen evolution in aqueous solution of methanol when films were irradiated with a 300-W Xe/Hg lamp. Copyright © 2013 American Chemical Society. (c) Average photocatalytic hydrogen evolution rates of CoFe2O4, CoFe2O4/Pt0.08Ag0.10, CoFe2O4/Pt0.08Ag0.12, and CoFe2O4/Pt0.08Ag0.14 under full-wave irradiation. (d) Cycling measurements of photocatalytic hydrogen production of Pt0.08Ag0.12/CoFe2O4. Copyright © 2021 Springer Nature Switzerland AG.
He et al. prepared nanoporous CoFe2O4 loaded with platinum and silver by dealloying [14][71]. They showed that the hydrogen precipitation rate of the resulting sample was as high as 2.36 mmol/h/g under full-spectrum irradiation, which was 24 times that of CoFe2O4 without platinum or silver (Figure 7c). The H2-releasing activity did not decrease significantly after 32 h of continuous irradiation (Figure 7d), indicating the excellent stability of this photocatalyst. The analysis showed that the silver NPs had a strong surface plasmon resonance (SPR) effect in visible light, resulting in effective visible light absorption. This effect expanded the range of light absorption and effectively improved light utilization, while Pt could act as an effective electron trap for electron–hole pair separation, effectively inhibiting electron and hole complexation. In addition, the simultaneous loading of Pt and Ag on CoFe2O4 produced a synergistic effect that contributed to its photocatalytic performance.
The morphological modification of photocatalysts and the construction of heterojunctions are considered to be the main means of significantly improving the performances of photocatalytic hydrogen evolution [15][16][72,73].
Zhang et al. successfully synthesized ZnS nanocrystals with different morphologies using cysteine as the sulfur source at different heating temperatures using a template-free method (Figure 8a,d) and evaluated the photocatalytic performances of the samples [17][74]. After a series of catalytic experiments, it was found that the inherent self-absorption and photon recirculation of photoluminescence played a key role in the photocatalysis (Figure 8e). The photocatalytic activity was investigated by the degradation of RhB solutions. It is well known that the morphology of a material has an important influence on its properties. As shown by the photocatalytic results (Figure 8f) and the morphological images, ZnS-200 exhibits a simpler surface structure and better catalytic activity than ZnS-100 and ZnS-150, suggesting the idea that defect-rich edge states are advantageous in providing reactive sites and broadening the absorption range.
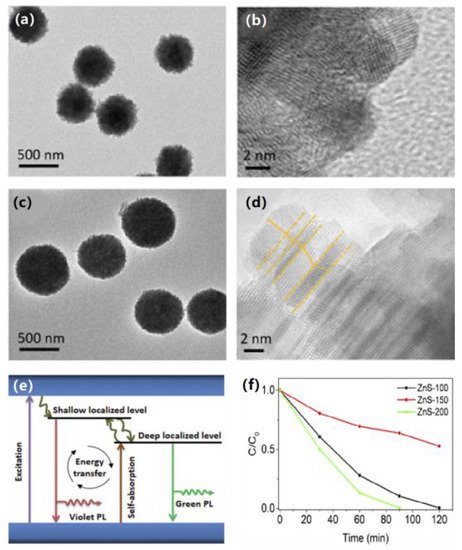
Figure 8. (a) TEM and (b) HRTEM images of ZnS-100. (c) TEM and (d) HRTEM images of ZnS-150. (e) Schematic illustration of electron transfer and proposed mechanism for photocatalytic performance. (f) Photocatalytic performances of samples for degradation of MB under xenon lamp irradiation. Copyright © 2020 Clarivate.
Bhirud et al. prepared hierarchical nanostructures of cubic-spinel-structured CdIn2S4 selectively by a hydrothermal method [18][75]. The effects of surfactants on the morphology and microstructure of cadmium sulfide were investigated using polyvinylpyrrolidone (PVP) and cetyltrimethylammonium bromide as surfactants. The cadmium sulfide samples prepared with PVP as the surfactant exhibited excellent photocatalytic activities, with a maximum hydrogen production rate of up to 3238 μmol/h.
3. Self-Assembled Nanocomposites for Energy Storage
3.1. Self-Assembled Nanocomposites for Lithium-Ion Batteries
Deng et al. reported a simple wet chemistry route for the large-scale synthesis of nearly monodispersed self-assembled SnO2 nanospheres by direct hydrogen peroxide oxidation of bulk tin (Sn) metal in deionized water (DIW) with the assistance of polyvinylpyrrolidone (PVP) and ethylenediamine (EDA) at room temperature, using PVP as a spatial stabilizer to limit the nanocrystal-to-nanocrystal contact, effectively preventing aggregation of nanocrystals [19][83] and causing particle aggregation to form nanospheres by minimizing the energy. The amount of PVP in the reaction system could lead to the controlled growth and self-assembly of SnO2 nanocrystals. IThis study contributed to the large-scale synthesis of self-assembled functional oxide nanostructures. Man et al. prepared SnO2 porous nanotubes (PNTs) by electrostatic spinning self-assembly [20](Figure 9a) [84]. The hollow structure and nano-SnO2 particles effectively increased the contact area between the electrolyte and the active material, alleviated the defects caused by volume expansion, and improved their electrochemical properties. It was found through testing that the SnO2-PNTs had excellent rate performances (Figure 9b). After charge/discharge tests, the coulombic efficiency exceeded 99% and could still provide a reversible capacity of 1045 mAh/g after 160 cycles (Figure 9c). As shown in the SEM images, there were no cracks on the electrode surface after cycling (Figure 9d), indicating its excellent structural stability.
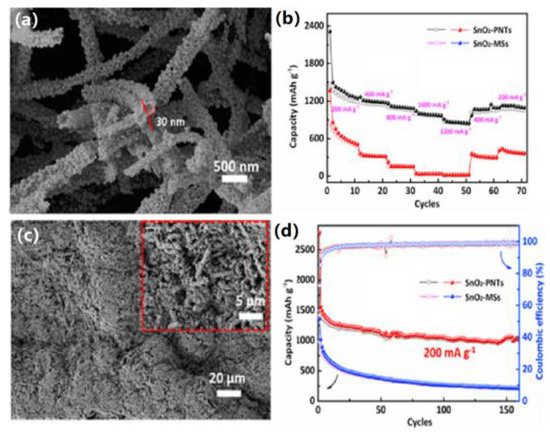
Figure 9. (a) SEM image of SnO2 porous nanotubes (SnO2-PNTs). (b) Rate performances of SnO2-PNTs and commercial SnO2 microspheres (SnO2-MSs). (c) SEM image of SnO2-PNTs after 100 cycles; the inset shows the partially enlarged view. (d) Cycling performances of SnO2-PNTs and SnO2-MSs at a current density of 200 mA/g. Copyright © 2008 American Chemical Society.
Molybdenum disulfide (MoS2) is a layered transition metal disulfide that has also attracted interest as an electrode material for lithium batteries due to its important mechanical, electrical, and optical properties. It has been found that the addition of carbon-based conductive additives to MoS2 can significantly improve the recyclability and testability of a material [21][22][85,86]. Das et al. prepared MoS2-carbon hierarchical nanostructures with different carbon compositions by hydrothermal self-assembly and investigated the application of the composite as a high-energy electrode for lithium-ion secondary batteries [23][87]. When the material was used as an electrode for lithium batteries, the binding of carbon provided significantly improved cycling stability and the carbon skeleton effectively inhibited particle agglomeration. Figure 10a shows that the MoS2-carbon nanocomposite exhibited excellent stability, with the MoS2-carbon nanocomposite containing 22% carbon (MS-22) showing the best stability.
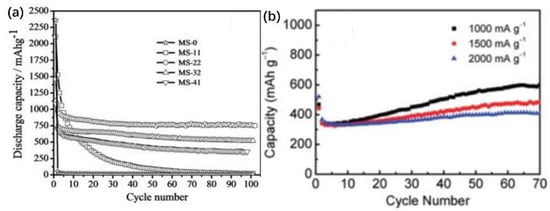
Figure 10. (a) Cycling stability of pure MoS2 and various MoS2-carbon composites. (b) Cycling performance of the MoO2/graphene composite electrodes in the range of 3–0.01 V vs. Li at current densities of 1000, 1500, and 2000 mA/g. Copyright © 2011 American Chemical Society.
Carbon coatings usually cover the surface of an active material tightly and do not effectively release the large strain from the volume expansion, which in turn leads to increased resistance for the lithium ions to reach the active material inside. Graphene is used as a nanostructured electrode material for energy applications due to its special structure, excellent electrical conductivity, large surface area, and chemical stability [24][88].
Sun et al. realized the large-scale preparation of MoO2/graphene nanocomposites by uniformly encapsulating MoO2 nanocrystals in graphene sheets and testing them as the positive electrodes of lithium-ion batteries. They found that the electrode material synthesized from MoO2/graphene nanocomposites had a significantly higher electrochemical performance than the bare MoO2 electrode material [25][89]. The electrochemical performance of the MoO2/graphene nanocomposite was found to be significantly higher than that of the bare MoO2 electrode material, with high cyclability and increasing reversible capacity. The coulomb efficiency approached 100% at high current densities, and the capacity reached up to 407.7 mAh/g after 70 cycles and up to 848.6 mAh/g at low current densities (Figure 10b). The morphology remained pristine, further demonstrating the high stability of the graded nanostructures, excellent cycling performances, and good rate capability.
MOFs or porous coordination polymers have received special attention as a new class of hybrid nanoporous materials because of their high surface areas and unique structure [26][27][90,91]. Zhu et al. prepared porous ZnO/Co3O4 nanocomposite clusters by self-assembly [28][92], with an initial discharge capacity of up to 2049 mAh/g, which showed high reversible capacity of 957 mAh/g after 100 cycles.
3.2. Self-Assembled Nanostructured Supercapacitor Materials
A composite of polycation-functionalized reduction of graphene oxide (FRGO-p) and MnO2 nanosheets (FRGO-p-MnO2) was synthesized by the electrostatic precipitation method [97] [29](Figure 11). Since the self-assembly of the MnO2 sheet effectively prevented the aggregation of MnO2, this material showed a strong capacitive performance and retained more than 89% of its initial capacitance after 1000 cycles. The layered nanostructure was prepared by an electrostatic self-assembly method [30][98]. The capacitance of the graphene supercapacitor was increased by more than 70%, the high-power density was increased by 15%, and the cycle life was increased. A graphene/carbon nanotube hybrid film with an interconnected carbon structure network was prepared by layer-by-layer (LBL) self-assembly technology [31][99]. The pure carbon electrode based on amine-based functionalized multi-walled carbon nanotubes (MWCNT-NH2) and rGO self-assembly prepared by Byon et al. had a specific capacitance of 120 F/g. The specific capacitance of the MWCNT/rGO electrode assembled by hydrazine steam treatment was about 1.5 times higher than that of rGO.
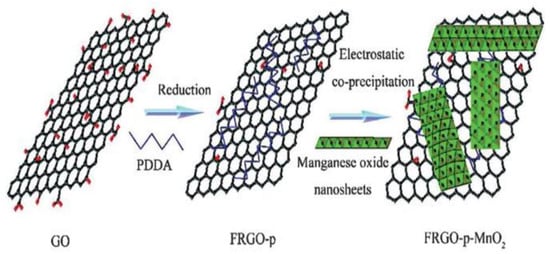
Figure 11. Schematic representation of the assembly process of FRGO-p-MnO2. Copyright © 2015 Elsevier Ltd. SMNs were fixed on the surface of rGO self-assembly and the resulting nanocomposites (SMN-RGO) could be used in supercapacitors. As shown in Figure 12a, the rectangular area of the cyclic voltammetry (CV) circuit in SmRGO2 (the theoretical mass ratio Sm2O3/GO is 1/1) was the largest. The specific capacitance (SC) value of the electrode calculated at a scanning rate of 50 mv/s was 227 F/g, which may have been caused by the synergistic effect between the components. Furthermore, the number of available active sites and the energy that could be stored at the supercapacitor electrode increased [100]. As shown in Figure 12b, the SCs of rGO, Sm2O3, SmRGO1 (the theoretical mass ratio Sm2O3/GO is 2/1), SmRGO2, and SmRGO3 (the theoretical mass ratio Sm2O3/GO is 1/2) decreased by varying degrees after 4000 cycles. The SC of SmRGO2 showed very high cycle stability, decreasing by 1.0% after 4000 cycles. Therefore, the application of the rGO dosage is very important to improve the cycle stability [100].
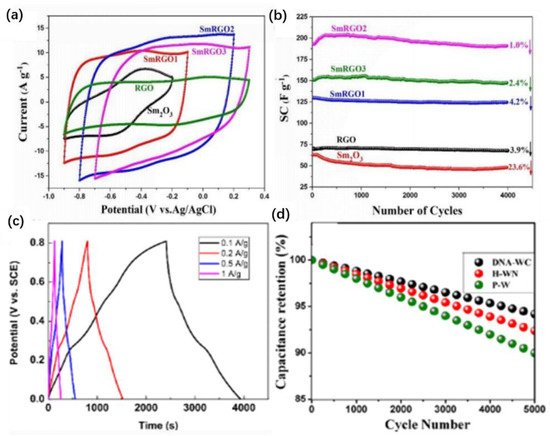
Figure 12. (a) Cyclic voltammograms of Sm2O3 nanoparticles SmN and SmN–reduced graphene oxide (SmN-RGO) electrodes with various ratios at 50 mV/s. (b) Specific capacitances of the RGO, SmN, and different SmRGOs as functions of the number of cycles at 200 mV/s. Copyright © 2017 Elsevier B.V. (c) Galvanostatic charge–discharge (GCD) curves of a high-density hybrid Na0.44MnO2 nanorod/active carbon films (HNAF) electrode. Copyright© 2021 Springer Nature Switzerland AG. (d) Overlay of cycling stability plots from GCD at a current density of 8 mA/cm2. Copyright © 2020 American Chemical Society.
A high-density hybrid film was prepared by self-assembly technology. The prepared activated carbon film could be used as the binder-free electrode of a supercapacitor. The constant current charge—discharge curve of the electrode (Figure 12c) showed that it had ideal charge—discharge characteristics and good reversibility [33][101].
Through the self-assembly of in situ carbon-fiber-coated WO3−x, a DNA-like double helix WO3−x/ultrafine fiber structure (DNA-WC) was designed. As an advanced supercapacitor material, it had excellent electrochemical properties, and its stability was greater than 94% (Figure 12d) after more than 5000 continuous cycles [34][102]. Stability is one of the important factors in the application of supercapacitors. The above materials have been studied in detail. With energy storage becoming a popular research subject, the application potential of supercapacitors in harsh environments has been deeply explored.
4. Self-Assembled Thin-Film Thermoelectric Materials for Energy Harvesting
4.1. Self-Assembled Thin-Film Thermoelectric Materials for Power Generation Using Waste Heat
Du et al. studied Sb-doped Mg2Si0.4Sn0.6 materials and reached a consistent conclusion, showing that the Seebeck coefficient increased by about 300 μV/K [35][36][105,106]. The thermoelectric figure of merit for Mg2.2Si0.7Sn0.3Sb0.01 was 0.64 at 723 K. Further studies of Sb-doped Mg2Si and Mg2Sn by Liu et al. showed a gradual increase in the Seebeck coefficient, with the thermoelectric figure of merit reaching about 1.3 at 700 K [37][107].
Polymer-based thermoelectric materials have been widely noticed for their good flexibility and low-density properties. To improve the energy conversion efficiencies of thermoelectric materials, polymer-based composites have become a popular research topic. Liu et al. prepared a functional film self-assembled from Bi2Se3 nanopillars using a solvothermal method [38][108], and the power factor of thermoelectricity was increased from 1.1 μW/cm·K2 of the sheet nanoflowers to 1.7 μW/cm·K2. The structures can significantly improve the performances of organic thermoelectric materials. Cho et al. prepared double-walled nanotubes (DWNT)-polyethyleneimine (PEI)/graphene-PVP multilayer nanocomposites using an LbL assembly method (Figure 13a), which uniformly bound active conducting elements into layered 3D hybrid organic nanostructures, significantly improving the electrical conductivity as well as the Seebeck coefficient [39](Figure 13b) [109]. Due to the increased conductivity of the nanocomposites, the power factor was also substantially increased, with a power factor of 1.9 μW/cm·K2. Te-based thermoelectric thin-film materials have attracted widespread interest due to their excellent thermoelectric properties. Zhou et al. prepared Te thin films by electrodeposition on stainless-steel substrates (SSS) with high flexibility. The room temperature power factor was 3.21 μW/cm·K2 and the thermal conductivity was 4.4 W/K·m [40][103].
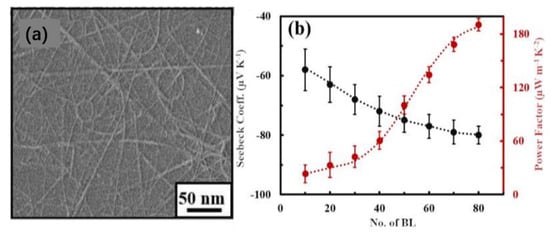
Figure 13. (a) SEM surface image of two bilayers (BL) DWNT-PEI/graphene-polyvinylpyrrolidone (PVP) on Si wafer. (b) Seebeck coefficient and power factor of DWNT-PEI/graphene-PVP. Copyright © 2021 Elsevier B.V.
