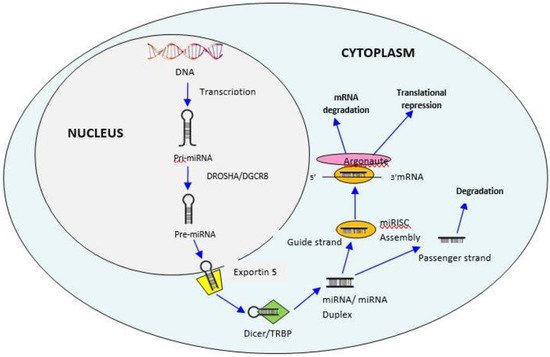
MiRNAs are significantly involved in several biological processes by regulation of gene expression. These are secreted in biological fluids like plasma, serum, cerebrospinal fluid, milk, urine, saliva, seminal fluid, tears and blood
[8]. These are called circulating or extracellular miRNAs. Plasma and serum miRNAs are found in different extracellular vesicles fluids such as exosomes, microvesicles, and apoptotic bodies based on their size and cellular origin. Extracellular vesicles are small membrane-bound particles released from many types of cells. Extracellular vesicles derived miRNA, including plasma miRNA, are highly stable and consist of proteins, lipids and nucleic acids
[24]. Many studies revealed that elevated release of extracellular vesicles from various cell types, including endothelial cells, RBCs and platelets, containing small RNAs, correlates with malaria severity
[25][26][25,26]. For example, the elevated level of extracellular vesicles derived miRNA-15-5p and miRNA-150b-5p were found in
Plasmodium vivax-infected patients while let-7a-5p was upregulated in both
Pv and
pf infected patients in Thailand
[27]. A recent study explored thatMiR-451 and miR16 are the most abundant miRNAs in normal plasma and normal RBCs
[24]. In contrast to another study, a significant decrease in the level of miRNA-16 and miRNA-145 in plasma of
Plasmodium vivax infected patients compared to normal individuals but no significant difference for
pf infected patients with normal individuals
[3][28][3,28]. Therefore, a diminished level of miRNA-145 leads to lower growth of the
Plasmodium parasite in in-vitro
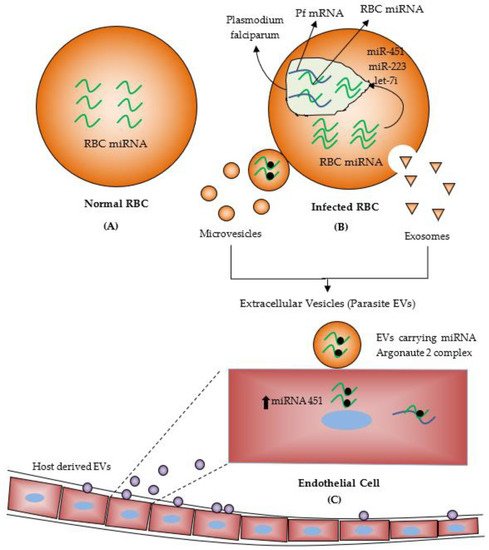
[29]. EVs released from infected RBCs carry functional miRNAs with Argonaute 2 complex protein in the bloodstream, and these EVs could be absorbed by the host endothelial cells. EVs delivered miRNAs alter the expression level of the target proteins and affect endothelial cell functions (
Figure 2)
[30]. Another study, Wang and colleagues suggested that more amounts of microparticles (extracellular vesicles) were released from a culture medium of malaria-infected RBCs than the normal RBCs. Human AGO 2 protein was found to bind with hundreds of miRNAs in these microparticlesand this miRNA-hAGO2, which is a component of the miRISC complex were transferred into the
Plasmodium falciparum [25]. AGO proteins (Argonaute proteins) are usually associated with small RNAs and are involved in post-transcriptional gene-silencing processes. When bound to miRNAs, AGO proteins also stabilize and protect miRNAs from degradation in animals and plants. Many studies also reported that EVs miRNAs could be transferred from one species to another and play crucial roles in cell-to-cell communication
[31].
Figure 2. (
A) About 200 miRNAs are found in human RBCs lacking nucleus and transcription/translation machinery. (
B)
Plasmodium-infected human red blood cells (RBCs). Transfer of miRNAs from RBC to
Plasmodium parasite and inhibit translation. (
C) Transfer of miRNAs from
Plasmodium-infected RBC to endothelial cells. (Reproduced from Bayer-Santos, E.; Marini, M. M.; da Silveira, J. F., Non-coding RNAs in host–pathogen interactions: subversion of mammalian cell functions by protozoan parasites. Frontiers in microbiology 2017, used under CC BY 4.0 with modification and addition of Normal RBCs, Microvesicles, Exosomes and Host derived EVs
[30]).
Several studies were also performed on an animal model for indicating miRNA expression in the malaria model. Cerebral malaria (CM) is a fatal complication of
Plasmodium infection, mostly affecting children. MiRNAs play an important role in regulating immune cell responses against infection. The infection of
Plasmodium chabaudi and
P.ANKA in the mice experiment model showed changes in the miRNA expression and caused the development of protective immunity against the
Plasmodium parasite. The up-regulation ofmiR-27a, miR-150 and let7i was seen in the brain of
Plasmodium berghei infected CBA mice that cause cerebral malaria when compared to non-cerebral malaria (NCB) and non-infected (NI) samples
[32]. Another study also analyzed the change in the expression profile of some miRNA by using the microarrays technique in microvesicles from
Plasmodium ANKA and
P.yoelii infected CBA mice that cause cerebral malaria and severe malaria and found miR-146a and miR-193b were rich in microvesicles from cerebral malaria-infected mice when compared with non-cerebral malaria and non-infected mice
[33]. MiRNAs may regulate biological pathways related to innate and adaptive immunity. Other studies analyzed dysregulated twelve, miRNAs. (MiR-21-5p,-18a-5p,-19a-3p, -20b-5p, -142-3p, -27a-5p, -152-3p, -193a-5p, -155-5p,-218-1-3p, -543, and -411-5p) between non-infected (NI) and cerebral malaria (CM). Three miRNAs, miR-142-3p, miR-27a-5p andmiR-19a-3p, were considerably upregulated in CM mice compared to NI and NCM. These miRNAs are significantly involved in some cerebral malaria-like adherens junctions, FoxO, TGF-β and endocytosis pathways
[34]. MiRNA-146a plays an important role in innate immunity responses, including IL-receptor-associated kinase (IRAK)-1 and TNF receptor-associated factor (TRAF)-6 (
Table 1)
[35].
Table 1. List of miRNAs studies conducted on humans and animal model experiments to explore various miRNA regulations and their functions.
| S. No |
Study/Author |
Year |
MiRNA |
Regulation |
Functions |
| 1. |
Ketprasit et al. [27] |
2020 |
MiR-150-5p andMiR-15b-5p |
Upregulated level of extracellular derived miRNA in | P. vivax | infected patient |
Adherens junction and TGF-beta signalling pathway. |
| |
|
|
let-7a-5p |
Upregulated in both | P. vivax | - and P. falciparum infected patients |
Adherens junction |
| 2. |
Aarón Martin-Alonso et al. [34] |
2018 |
miR-19a-3p, miR-27a-5p, and miR-142-3p |
It is upregulated in CM infected mice’s brains compared to NI and NCM. |
Play a significant role in several pathways relevant to CM, including the TGF-β and endocytosis pathways. |
| 3. |
Cohen et al. [33] |
2018 |
miR-146a and miR-193b |
Upregulated in microvesicles from cerebral malaria-infected mice |
Cerebral pathology |
| 4. |
Chamnanchanunt et al. [3] |
2015 |
MiR-145 and miR-16 |
Down-regulated in serum of | P. vivax | infected patients |
Not defined |
| |
|
|
miR-223, miR-226-3p |
No change |
Not defined |
| 5. |
LaMonte et al. [19] |
2012 |
MiRNA-145, MiRNA-223 and let-7i |
It is upregulated in HbAS and HbSS erythrocytes of P. falciparum infected patients. |
Integrated into parasite mRNAs and resulted in translational inhibition. |
| 6. |
El-Assaad et al. [32] |
2011 |
MiR-27a, miR-150 |
Upregulated in the brain tissue of PbA infected mice |
Cell proliferation, development, and differentiation. |
| |
|
|
let-7i |
Upregulated in the brain tissue of PbA infected mice |
Cellular proliferation and the innate immune response |
| 7. |
Rathjen et al. [20] |
2006 |
MiR-145 |
Upregulated in both infected and healthy red blood cells |
Differentiation of erythroid cells. |