Although stem cells have been considered promising for the treatment of degenerative diseases by ‘seeding’ them into damaged tissues, it has recently been observed that the regenerative capacity of stem cells is influenced and regulated by the local immune response and in particular by macrophages, which constitute a central component of the damage response and are the coordinators of tissue repair and regeneration. Among the panoply of immune cells involved in the response to both acute and chronic wounds, recent discoveries have highlighted novel and often unexpected roles for certain types of immune cells in promoting a permissive local environment for effective cell replacement and restoration of tissue integrity. Some studies have shown that the control of inflammation is crucial in regenerative therapies: To be effective, regenerative therapies must block and control inflammation to allow tissue regeneration by resident stem cells. Indeed, the presence of inflammation inhibits the regenerative action of tissue-resident mesenchymal stem cells (MSCs). Recent papers suggest that an innovative regenerative strategy could be to polarize macrophages from the M1 inflammatory state to the M2 anti-inflammatory state utilizing immune cells. These reviews conclude that next-generation regenerative therapies need an immune-centric approach instead of the use of stem cells. Thus, depending on the tissue or organ targeted, regenerative strategies could be developed to stimulate macrophage polarization or to recruit subpopulations of pro-healing macrophages. Already, Mordechait has been observed in 2013 and Pinto in 2014 (7) have shown that the regeneration of myocardial tissue after ischemia was induced by macrophages that regulate resident stem cells and promote regeneration, suggesting that targeting macrophages could be a new strategy to improve infarct healing and repair. The regenerative and stem-cell-controlling capacity of macrophages has also recently been demonstrated in bone tissue: by Gibon et al., Gullard et al., and Ekstrom. Najar et al. in 2018 clarified that mesenchymal stem cells act through a paracrine and immune-modulatory and non-differentiative mechanism and that the microenvironment and immune system regulate the activity of MSCs regardless of the tissue from which they originate. Based on the role played by several types of macrophages and lymphocytes in the wound-healing response, it is tempting to hypothesize that interventions that reduce the M1 macrophage phenotype and promote M2 may represent a new therapy to induce tissue regeneration.
- wound healing
- diabetic foot
- immune system
- monocytes
- lymphocytes
- immune centric revolution
1. How to Switch to M2 Regenerative Phenotype?
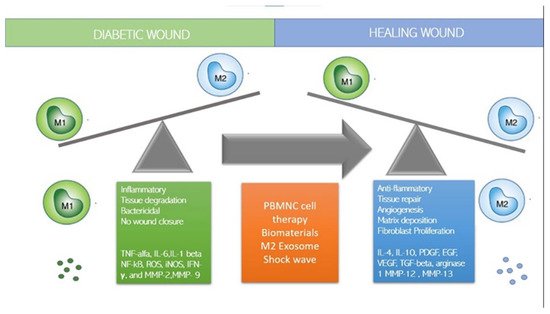
2. Immune-Cell-Based Cell Therapy
| Description | Result | Ref. | |
|---|---|---|---|
| Zuloff-Shani et al. 2004 | Treatment of chronic ulcers with blood-derived macrophages activated by hypo-osmotic shock in over 1000 patients | Reduction of the healing time, reduction of risk of complications and morbidity. Improvement of the quality of life for long-suffering patients | [23] |
| Danon et al. | Decubital ulcers of 72 patients (average age 82), were treated by local injection of macrophages prepared from a blood unit in a closed sterile system. The remaining 127 patients (average age 79) were treated conventionally and served as controls. No exclusion criteria were applied. | In the macrophage-treated group, 27% healed, while only 6% healed in the control group (p < 0.001). Moreover, the macrophage-treated group showed a faster healing (p < 0.02) | [24] |
| Zuloff-Shani et al. 2010 | 100 consecutive elderly patients with a total of 216 stage III or IV pressure ulcers, 66 patients were assigned to the autologous macrophages group, 38 patients were assigned to the standard care treatments (38 patients.) | Percentage of completely closed wounds (wound level and patient level) were significantly better (p < 0.001/p < 0.001, respectively) in all patients in favor of AMS, as well as in the subset of diabetic patients (p < 0.001/p < 0.001). | [25] |
| Moriya, J et al. | Retrospective study on 42 patients with severe intermittent claudication, ischemic rest pain, or non-healing ischemic ulcers caused by peripheral arterial disease, including thromboangiitis obliterans, and who had not responded to conventional therapy that included nonsurgical and surgical revascularization (no option). | Improvement of ischemic symptoms was observed in 60% to 70% of the patients. The annual rate of major amputation was decreased significantly by treatment. The survival rate of younger responders was better than that of non-responders. | [26] |
| Huang, P.P et al. | 150 patients with peripheral arterial disease were randomised to mobilized PBMNC 76 cases or BMMNC 74 cases implanted, follow up for 12 weeks. Primary outcomes were safety and efficacy of treatment, based on ankle-brachial index (ABI) and rest pain | Significant improvement of the ABI, skin temperature and rest pain was observed in both groups after transplantation and was better I PBMNC group. However, there was no significant difference between two groups for pain-free walking distance, transcutaneous oxygen pressure, ulcers, and rate of lower limb amputation | [27] |
| Liotta, F et al. | Autologous Non-Mobilized Enriched Circulating Endothelial Progenitors obtained from non-mobilized peripheral blood by immunomagnetic selection of CD14+ and CD34+ cells) or BM-MNC were injected into the gastrocnemius of the affected limb in 23 and 17 patients with no option critical limb ischemia. | After 2 yrs follow-up, both groups showed significant and progressive improvement in muscle perfusion (primary endpoint), rest pain, consumption of analgesics, pain-free walking distance, wound healing, quality of life, ankle-brachial index, toe-brachial index, and transcutaneous PO2 | [28] |
| Dubsky, M et al. | 28 patients with diabetic foot disease (17 treated by bone marrow cells and 11 by peripheral blood mononuclear cell) were included into an active group and 22 patients into a control group without cell treatment. | The transcutaneous oxygen pressure increased significantly (p < 0.05) compared with baseline in both active groups after 6 months, with no significant differences between bone marrow cells and peripheral blood cell groups, while no change in the control group was observed. The rate of major amputation by 6 months was significantly lower in the active cell therapy group compared with that in the control group (11.1% vs. 50%, p = 0.0032), with no difference between bone marrow cells and peripheral blood cells. | [29] |
| Persiani, F. et al. | 50 diabetic patients affected by CLI underwent PBMNCs implant (32 patients underwent PBMNCs therapy associated with endovascular revascularization, 18 patients, non-option CLI) | The follow-up period was 10 months. In the PBMNC group + revascularization TcPO, pain VAS Scale improved. In PBMNCs therapy group, the mean TcPO2 improved from 16.2 ± 7.2 mmHg to 23.5 ± 8.4 mmHg (p < 0.001), and VAS score means decreased from 9 ± 1.1 to 4.1 ± 3.3 (p < 0.001). Major amputation was observed in 3 cases (9.4%), both in adjuvant therapy group and in PBMNCs therapy. (16.7%) (P ¼ 0.6) as the therapeutic choice (PBMNCs therapy group). |
[30] |
| De Angelis, B et al. | Prospective, not randomized study based on a treated group who did not respond to conventional therapy (n = 43) when implanted with A-PBMNC cells versus a historically matched control group. Patients of both groups were suffering from CLI Fontaine scale IV with chronic ulcers |
The A-PBMNC-treated group showed a statistically significant improvement of limb rescue of 95.3% versus 52.2% of the control group (p < 0.001) at 2 years. The A-PBMNC group also showed reduction in pain at rest, increased maximum walking distance, and healing of the wound and an overall improvement in the quality of life. Post-treatment radiological studies showed an improvement of vascularization with the formation of new collateral and by histological findings. | [31] |
| Dubsky et al. | 31 patients with DFU and CLI treated by autologous stem cells and 30 patients treated by PTA were included in the study; 23 patients with the same inclusion criteria who could not undergo PTA or cell therapy formed the control group. |
Amputation-free survival after 6 and 12 months was significantly greater in the cell therapy and PTA groups compared with controls (p < 0.001 and p < 0.0029, respectively) without significant differences between the active treatment groups. Increase in TcPO2 did not differ between cell therapy and PTA groups until 12 months but TcPO2 in the control group did not change over the follow-up period. More healed ulcers were observed up to 12 months in the cell therapy group compared with the PTA and control groups (84% vs. 57.7% vs. 44.4%; p < 0.042). |
[32] |
| Scatena et al. | The study included 76 NO-CLI patients with DFUs. All patients were treated with the same standard care (control group), but 38 patients were also treated with autologous PBMNC implants. |
Only 4 out 38 amputations (10.5%) were observed in the PBMNC group, while 15 out of 38 amputations (39.5%) were recorded in the control group (p = 0.0037). The Kaplan–Meier curves and the log-rank test results showed a significantly lower amputation rate in the PBMNCs group vs. the control group (p = 0.000). At two years follow-up, nearly 80% of the PBMNCs group was still alive vs. only 20% of the control group (p = 0.000). In the PBMNC group, 33 patients healed (86.6%) while only one patient healed in the control group (p = 0.000). |
[33] |
| Di Vieste et al. | Case report of a 59-year-old patient with type 2 diabetes mellitus who had a gangrene of the right toe. After an ineffective angioplasty, it was decided to use a PBMNC therapy. | The patient underwent to amputation of the first necrotic toe and three PBMNC treatment sessions with complete surgical wound healing and limb rescue |
3. Mesenchymal Stem Cells (MSC)
4. Extracellular Vesicles (EVs) and Exosome (Exo)
5. Dermal Substitutes
| Primary Material Composition |
Source and Other Components | Refs. | |
|---|---|---|---|
| Nevelia | Porous resorbable double layer matrix 2 mm thickness made of stabilized native collagen type I and a silicone sheet 200 mm thickness mechanically reinforced with a polyester fabric. The extraction procedure and the freeze-drying process allow the structuring of the collagen into a matrix with optimal hydrophilicity, pore structure and pore size (20–125 μm) |
Bovine, Native collagen Type I. No glycosaminoglycan (GAG) added to improve cell attachment and proliferation. Glutaraldehyde Cross-linking |
[58][59][60] |
| Integra | Bilayer system for skin replacement made of a porous matrix of fibers of cross-linked bovine tendon collagen and glycosaminoglycan (chondroitin-6-sulfate) that is manufactured with a controlled porosity and defined degradation rate. The Integra pore size of 20 to 125 μm allows influx of cells. |
Bovine Tendon Type I Collage Shark cartilage -derived chondroitin-6-sulphate (GAG). Glutaraldehyde Cross-linking |
[61][62][63] |
| PriMatrix | Acellular dermal tissue matrix. comprising of both type I and type III collagen derived from fetal bovine dermis. This matrix is processed in a way to maintains the extracellular matrix in its native and undamaged state while removing all lipids, fats, cells, carbohydrates and non-collagenous proteins. |
Fetal Bovine collagen type I and type III collagen. No cross-link |
[62] |
| Oasis Wound Matrix |
Lyophilized, decellularized porcine small intestine submucosa (SIS). Matrix is derived from a single layer of porcine small intestinal submucosa (SIS) technology. The technology provides an intact three-dimensional extracellular matrix which allows for host cell migration. The SIS is freeze-dried and sterilized with ethylne oxide gas in preparation for clinical use |
Porcine small intestine submucosa (SIS). No cross-link |
[62] |
| Allomend | Decellularized donated human dermal tissue, with significant removal of cellular debris (including DNA and RNA), proteins and antigens. The process does not require the use of detergents or enzymes, thereby mitigating the possibility of harmful residuals in the tissue. The decellularization process also inactivates microorganisms through cellular disruption. USA only, not available in Europe |
Human dermal tissue No cross link |
[61][62][63] |
| DermaMatrix | Cadaveric human allograft treated with a disinfectant solution that combines detergents with acidic and antiseptic reagents. USA only, not available in Europe |
Human dermal tissue No cross link |
[61][62][63] |
| Dermacell | Decellularized regenerative human tissue matrix allograft processed using proprietary technology that removes at least 97% of donor DNA without compromising the desired biomechanical structure or biochemical properties. USA only, not available in Europe |
Human dermal tissue No cross link |
[61][62][64] |
