Camelina (Camelina sativa (L.) Crantz) is a protein and oilseed crop belonging to the family Brassicaceae.
- camelina
- poultry
- n-3 fatty acids
- meat quality
1. Introduction
| Parameters | Seed | Cake | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| References | |||||||||||||||
| [15] | [18] | [32] | [19] | [33] | [20] | [34] | [21] | [8] | [7] | [35] | [22] | [36] | [23] | ||
| Metabolizable energy, MJ kg | −1 | 14.13 | 17.88 | - | 9.11 | - | - | - | |||||||
| Dry matter, % | 93.66 | 92.02 | 95.81 | 93.45 | 91.95–92.10 | 90.89 | |||||||||
| Crude protein, % | 24.78 | 37.17 | 34.99 | 36.88 | 33.31 | 34.25–34.40 | 39.8 | ||||||||
| Ether extract, % | 36.84 | 19.17 | 13.55 | 6.44 | 16.91 | 21.00–22.71 | 12.7 | ||||||||
| Crude fiber, % | 11.40 | 10.72 | 9.90 | 17.40 | 10.53 | 9.37 | 12.0 | ||||||||
| Crude ash, % | 4.27 | 6.80 | 5.67 | 5.97 | 4.91 | 5.01–5.38 | 6.30 | ||||||||
| Neutral-detergent fiber, % | - | 35.63 | - | 45.50 | - | 26.32–26.63 | 38.30 | ||||||||
2. Camelina Chemical Composition
2.1. Camelina Fatty Acid Composition
The content of total n-3 PUFA, which is always deficient in standard poultry feeding, is from 3.11 to 4.00, 2.05 to 3.96 and 1.0 to 2.16 times higher in camelina cake and seed, than that found in soybean meal, rapeseed cake and hempseed cake, respectively, but from 1.50 to 2.19 times lower than in linseed cake [50,52,59,60][34][40][43][44]. Camelina cake and oil has a lower n-6/n-3 PUFA ratio in comparison with soybean meal, rapeseed cake and hempseed cake, respectively, 0.60–0.99 vs. 6.10, 1.68, 4.08 [37,51,52][24][39][40]. The linoleic/α-linolenic ratio in camelina cake (0.72) was also lower compared with rapeseed cake (1.66), hempseed cake (3.76) and soybean meal (6.11) [51,52,53][39][40][38].
2.2. Antioxidant Content in Camelina
2.3. Antinutritive Compounds in Camelina
The use of camelina feedstock in poultry nutrition is limited by plant secondary metabolites, i.e., glucosinolates, sinapine, phytic acid and condensed tannins that are ascribed to antinutritive compounds found in camelina.
Glucosinolate accumulation in camelina depends on many factors—genotype, climatic conditions, soil type, sulfur content in the soil, and fertilization [14,71][13][51]. Therefore, a wide range of glucosinolate content can be found in camelina. The content of glucosinolates in whole seed, as reported by Schuster and Friedt [71][51], varies from 13.2 to 36.2 μmol/g and the mean value is 24 μmol/g; while Matthäus and Zubr [14][13] indicated it ranges from 9 to 19 μmol/g. The amount of glucosinolates in camelina cake is from 14.5 to 44.9 μmol/g [14,36,46,72,73,74][13][23][30][52][53][54]. Matthäus and Zubr [14][13] indicated that glucosinolates are stored in the residue when oil is produced during seed pressing. Research data show that whole seed contains 14.1 μg/mg, seed meal 24.3 μg/mg, and defatted meal as much as 31.8 μg/mg glucosinolates [75][55].
Woyengo et al. [36][23] indicate that poultry can tolerate up to 2.0 μmol/g glucosinolates in rapeseed diets, while Tripathi and Mishra [68][56] increase the tolerance level to 5.6 μmol/g. No sufficient research data can be found to define the effects of camelina-specific glucosinolates and their metabolic products on poultry nutrition.
Since the amount of glycosinolates in camelina varieties varies widely, it indicates a high phenotypic variation, which is a prerequisite for successful selection. Currently, the major breeding objectives for camelina are to increase seed yield, seed oil and protein content, and resistance to abiotic stress, however, varieties with low glucosinolate levels have not been developed.
The content of sinapine varies markedly in camelina plants. The seed analysis of eight different camelina genotypes indicated the range of sinapine content to be from 2.8 to 7.8 mg/g, with an average of 4 mg/g [76][57]. Meanwhile, the analysis of 30 camelina cultivars from different European localities showed that sinapine concentration in oilseed cake is from 1.7 to 4.2 g/kg [14][13]. A similar mean sinapine content is found in camelina cake by other researchers, i.e., 2.32 g/kg [72][52], 2.57 g/kg [74][54] and 2.79 g/kg [73][53]. The content of sinapine in camelina is much lower than that found in other Brassicaceae family plants such as rape or mustard (7 and 13 mg/g, respectively) [14][13]. Feedstuffs with sinapine taste bitter, but as taste buds in birds are poorly developed [79][58], feed bitterness does not reduce voluntary feed intake in broilers [80][59]. However, if no more than 10% of camelina cake is used in meat poultry diets, no undesirable sinapine effect will be found, due to a low sinapine concentration.
3. Influence of Camelina on Growth Performance
| Poultry/Feed | Level, % | Trial Period, Days | Body Weight, g | Weight Gain, g | Feed Intake, g/Birds | Feed Conversion Ratio, kg/kg | Bird Mortality, % | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Chicken/cake | 2.5 | 1–42 | −172.29 * | −173.59 * | +2.3 | +0.17 | - | [7] | [6] |
| 5 | +54.37 | +54.43 | +312.8 | +0.11 | - | ||||
| 10 | −59.69 | −59.01 | +182.1 | +0.15 | - | ||||
| Chicken/oil, cake | Oil, 4 | 22–42 | - | −22 | −50 | −0.04 | +0.66 | [85] | [65] |
| Cake, 10 | - | −122 | −116 | +0.09 | +0.75 | ||||
| Chicken/oil, seed | Oil, 2.5 | 11–42 | +63.82 | - | +87.8 | −0.01 | −0.38 | [15] | [18] |
| Seed, 5 |
4. Influence of Camelina on PUFA Composition in Breast, Leg Muscles and Liver
| Poultry/ Feed |
Level,% | Trial Days | C18:3 | C20:3 | C20:5 | C22:5 | C22: 6n-3 |
LC n-3 PUFA | n-3 PUFA | C18: 2n-6 |
n-6 PUFA | n-6/n-3 PUFA | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast | |||||||||||||||||||||||
| Chicken/ cake |
8 | 1–42 | +2.1 * | +0.12 * | +0.01 | +0.26 | 0 | +0.3 | +2.3 * | +1.6 * | +1.4 | −1.2 * | [97] | [82] | |||||||||
| 16 | +3.4 * | +0.33 * | −0.03 | +0.58 * | +0.1 | +1 * | +4.3 * | +3.4 * | +3.4 * | −1.5 * | |||||||||||||
| 24 | +4.8 * | +0.37 * | 0 | +0.64 * | +0.1 | +1.1 * | +5.7 * | +5.1 * | +5.4 * | −1.6 * | |||||||||||||
| Chicken/ cake |
2.5 | 1–42 | +0.59 * | - | +0.03 | +0.21 | +0.34 | - | +1.16 * | +0.48 | −1.88 | −6.4 * | [7] | [6] | |||||||||
| 5 | +0.95 * | - | +0.08 | +0.33 | +0.28 | - | +1.62 * | +1.85 | −1.07 | −7.62 * | |||||||||||||
| −31.86 | - | +134.85 | +0.08 | +0.39 | |||||||||||||||||||
| 10 | +1.62 * | - | +0.14 | +0.12 | +0.22 | - | +2.10 * | −2.37 | −8.5 * | −10.44 * | Seed, 10 | ||||||||||||
| Chicken/ | −188.29 * | oil, cake | - | −40.44 | Oil, 4 | 22–42 | +4.5 * | +0.13 | +0.19 | ||||||||||||||
| - | - | - | - | - | +4.88 * | +0.16 | −0.68 | −2.04 * | [ | 85 | ] | [ | 65] | Quail/cake | 5 | 1–35 | +2.65 | +2.65 | +7.71 | −0.00001 | - | [34 | |
| Cake, 10 | +3.69 * | - | ] | - | [ | 21 | ] | ||||||||||||||||
| - | - | - | +3.73 * | +1.49 * | +0.51 | −1.66 * | 10 | +1.06 | +0.94 | +10.9 | +0.00004 | - | |||||||||||
| Chicken/ oil, seed |
Oil, 2.5 | 11–42 | +2.46 * | - | +0.42 * | +0.19 * | +0.48 * | - | +3.50 * | +0.83 | −2.36 * | - | [15] | [18] | 15 | +3.35 | +2.38 | +31.27 | +0.00013 * | ||||
| Seed, 5 | - | ||||||||||||||||||||||
| +1.73 * | - | +0.15 * | +0.16 * | 20 | −2.31 | −3.53 | +17.92 | +0.00017 * | - | ||||||||||||||
| Turkey/cake | 5 | 1–28 | −32 | - | +19 | +0.09 | [86] | [66] | |||||||||||||||
| - | +0.54 * | - | +3.36 * | +1 | +1.3 * | −2.24 * | [ | 98 | ] | [ | 83 | ] | 15 | −56 | - | +4 | +0.12 | - | |||||
| +0.40 * | - | +2.37 * | +1.30 | −1.47 * | - | ||||||||||||||||||
| Seed, 10 | +2.38 * | - | +0.55 * | +0.25 * | +0.46 * | - | +3.55 * | +0.59 | −2.41 * | - | |||||||||||||
| Chicken/ cake |
5 | ||||||||||||||||||||||
| 10 | +3.63 * | - | +0.52 * | - | +0.36 * | - | +5.33 * | +1 | +1.0 * | −2.23 * | 5 | 1–28 | −66 | - | −90 | +0.03 | - | [86 | |||||
| 15 | +3.56 * | - | +0.54 * | ] | [ | 66 | ] | ||||||||||||||||
| - | +0.76 * | - | +6.03 * | +1.5 | +2.3 * | −2.33 * | 15 | −154 * | - | −226 * | +0.06 | - | |||||||||||
| 1–38 | +1.72 * | ||||||||||||||||||||||
| 20 | +4.72 * | - | +1.01 * | - | +0.86 * | - | +8.33 * | 0 | +0.7 * | −2.38 * | 20 | −216 * | - | −197 * | +0.30 * | - | |||||||
| 25 | +5.07 * | - | +1.08 * | - | +1.78 * | -- | +10.43 * | +2 | +3.4 * | −2.88 * | Chicken/oil | ||||||||||||
| Duck/ | 6.91 4.07 |
1–21 22–35 |
- | −10 | +50 | +0.03 | - | cake | [37] | [24 | 15–20 | ] | |||||||||||
| 1–49 | +1.49 * | +0.08 * | +0.08 | +0.09 | +0.13 | +1.86 * | +0.28 | +0.01 | −2.16 * | [ | 52 | ] | [40] | In comparison with soybean oil | |||||||||
| Chicken/ oil |
6.91 4.07 | 1–21 22–35 |
+3.07 * | - | - | - | - | - | - | −21.27 * | - | −35.14 * | [37] | [24] | - | −70 | −170 | −0.03 | - | ||||
| In comparison with soybean oil | In comparison with rapeseed oil | ||||||||||||||||||||||
| +3.55 * | - | - | - | - | - | - | +4.13 * | - | −17.82 * | Chicken/seed | 10 | 7–42 | −116.8 * | −122.13 * | −250 | +0.01 | +0.5 | [87 | |||||
| In comparison with rapeseed oil | ] | [ | 67 | ] | |||||||||||||||||||
| Chicken/oil | 3 | 22–49 | +61 | - | - | −0.03 | +0.09 | [88] | [68] | ||||||||||||||
| 6 | +76 | - | |||||||||||||||||||||
| Leg | - | −0.04 | +2.62 | ||||||||||||||||||||
| Chicken/cake | 8 | 1–42 | +1.7 * | +0.08 * | −0.01 | +0.18 * | +0.07 | +0.33 | +2.1 * | −0.9 | −1.4 | −3 * | [97] | [82] | Chicken/cake | 10 | 1–21 | −60 * | - | −71 * | + 0.03 | - | [ |
| 16 | +4.2 * | +0.20 * | 0 | +0.21 * | 33 | ] | [ | 20] | |||||||||||||||
| - | +0.32 * | +0.06 | +0.47 * | +4.7 * | +2.5 | +2.6 | −2.9 * | Chicken/cake | 8 | 23–42 | −35.69 | −22.17 | −17.5 | + 0.01 | - | [ | |||||||
| 24 | +6.4 * | +0.23 * | 0 | 53 | ] | [ | 38 | ] | |||||||||||||||
| +0.27 * | +0.14 | +0.63 * | +7.1 * | +3.9 * | +3.6 | −3.6 * | Chicken/cake | 10 | 1–42 | +107.5 | +3.13 | +0.10 | - | [89] | [69 | ||||||||
| Chicken/cake | 2.5 | 1–42 | +0.37 | - | ] | ||||||||||||||||||
| +0.08 | +0.37 * | +0.38 * | - | +1.2 * | −2.44 | −1.04 | −9.38 * | [ | 7 | Chicken/cake | 5 male | 1–37 | −215 * | - | - | - | - | [8] | [7] | ||||
| 5 female | −67 | - | - | - | - | ||||||||||||||||||
| ] | [ | 6 | ] | ||||||||||||||||||||
| 5 | +0.61 * | - | +0.04 | +0.33 * | +0.25 * | - | +1.23 * | −2.58 | −1.84 | −9.83 * | |||||||||||||
| 10 | +1.45 * | - | +0.23 * | +0.42 * | +0.40 * | - | +2.5 * | −3.93 | −3.81 | −13.91 * | 10 male | −264 * | |||||||||||
| Chicken | - | - | cake | - | - | ||||||||||||||||||
| female | 5 | 1–37 | +1.48 * | - | - | - | - | - | +1.55 * | −0.16 | +0.04 | −1.11 * | [8] | [7] | 10 female | −128 * | - | - | |||||
| 10 | +4.02 * | - | - | - | |||||||||||||||||||
| - | - | - | - | +4.17 * | +1.79 * | +2.16 * | −1.89 * | 5 | |||||||||||||||
| Chicken cake male | 1–14 | - | - | −3 * per day | - | - | |||||||||||||||||
| 5 | 1–37 | +1.88 * | - | - | - | - | - | +2.05 * | +0.62 | +0.68 | −1.46 * | 10 | - | - | −4.3 * per day | - | - | ||||||
| 10 | +4.53 * | - | - | - | - | - | +4.69 * | +1.63 * | +1.56 | −2.39 * | 5 | 15–37 | - | - | −3 per day | - | - | ||||||
| Duck/ cake |
15–20 | 1–49 | +2.33 * | +0.10 * | +0.06 | +0.04 | +0.06 | - | +2.5 * | +0.29 | +0.02 | −2.94 * | [52] | [40] | 10 | - | - | −5 per day | - | ||||
| Chicken/ | - | ||||||||||||||||||||||
| oil | 6.91 4.07 |
1–21 22–35 |
+7.59 * | - | - | - | - | - | - | −7.90 * | - | −11.57 * | [37] | [24] | 5 | 1–37 | - | - | - | +0.05 * | - | ||
| In comparison with soybean oil | 10 | - | - | - | +0.08 * | - | |||||||||||||||||
| +8.45 * | - | - | Chicken/cake | 8 | 1–42 | +334.5 * | +8 * | +1.7 | - | +0.54 | [35] | [22] | |||||||||||
| 16 | +508.5 * | +12.2 * | +0.7 | - | +11.29 * | ||||||||||||||||||
| 24 | +105.6 | +2.6 * | −1 | - | +12.89 * | ||||||||||||||||||
| - | ||||||||||||||
| - | ||||||||||||||
| - | ||||||||||||||
| - | ||||||||||||||
| +10.44 * | ||||||||||||||
| - | ||||||||||||||
| −9.43 * | ||||||||||||||
| In comparison with rapeseed oil | ||||||||||||||
| Liver | ||||||||||||||
| Chicken/ | ||||||||||||||
| cake | ||||||||||||||
| 8 | ||||||||||||||
| 1–42 | ||||||||||||||
| +0.47 * | ||||||||||||||
| +0.08 * | ||||||||||||||
| +0.02 | ||||||||||||||
| +0.21 * | +0.50 * | +0.8 * | +1.3 * | +2.2 * | +2.3 * | −1.2 * | [ | 97 | ] | [ | 82 | ] | ||
| 16 | +1.13 * | +0.19 * | +0.03 | +0.59 * | +1.24 * | +2.0 * | +3.2 * | +4.8 * | +6.5 * | −1.7 * | ||||
| 24 | +2.10 * | +0.37 * | +0.03 | +0.97 * | +2.63 * | +3.9 * | +6.1 * | +9 * | +12.4 * | −2.2 * | ||||
| Chicken /cake |
2.5 | 1–42 | −0.25 | - | +0.21 * | +0.80 * | 0 | - | +2.58 * | −7.91 * | −2.83 | −6.45 * | [7] | [6] |
| 5 | −0.21 | - | +0.31 * | +0.64 | +3.05 * | - | +3.79 * | −7.77 * | −3.86 * | −7.66 * | ||||
| 10 | +0.43 | - | +0.45 * | +1.19 * | +4.02 * | - | +6.09 * | −5.01 | −3.59 * | −8.85 * | ||||
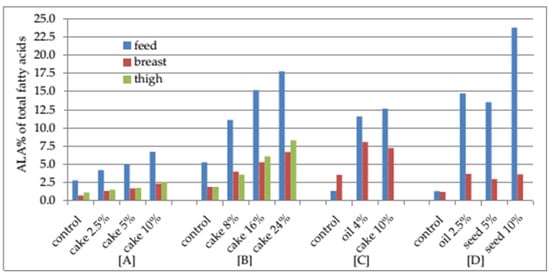
5. Conclusions
Camelina seed and its by-product from oil or biodiesel production, such as cake, can be used for meat poultry feeding because they are a valuable feed rich in crude protein (25–40%), oil (6–37%) and antioxidant substances. The content of crude protein and composition of camelina amino acids is close to that of rapeseed meal.
Camelina is distinguished by a unique fatty acid composition, as ALA accounts for 25.88 to 36.67% of the total fatty acids.
However, camelina also contains antinutrients, especially glucosinolates, that prevent the use of seed and its by-products in poultry nutrition on a larger scale.
Addition of camelina seed, oil, cake to poultry diets results in 1.32 to 7.23 times higher ALA content in chicken muscles, in comparison with conventional chicken diets. Consequently, higher ALA content reduces the n-6/n-3 PUFA ratio from 1.32 to 8.35 times in muscles.
Poultry with a higher n-3 PUFA content is beneficial to consumers in their pursuit of healthier products, as such meat increases the consumption of currently deficient n-3 PUFAs and consequently lowers the risks of cardiovascular diseases. Moreover, the use of camelina cake in poultry diets lowers the cost price of poultry and enhances the sustainability of poultry growing and biofuel production. A wider use of camelina should reduce, at least partly, the dependence on imported non-sustainable soya bean meal, and induce its cultivation worldwide, thus, increasing the crop variety used in agriculture.
References
- Denanot, J.P. REPORT on a European Strategy for the Promotion of Protein Crops—Encouraging the Production of Protein and Leguminous Plants in the European Agriculture Sector (2017/2116(INI)). Committee on Agriculture and Rural Development. European Parliament 2014–2019. 2018. Available online: https://www.europarl.europa.eu/doceo/document/A-8-2018-0121_EN.html (accessed on 2 December 2020).
- Mozaffarian, D.; Ascherio, A.; Hu, F.B.; Stampfer, M.J.; Willett, W.C.; Siscovick, M.D.; Rimm, E.B. Interplay Between Different Polyunsaturated Fatty Acids and Risk of Coronary Heart Disease in Men. Circulation 2005, 111, 157–164.
- Thiebaut, A.C.M.; Chaje‘s, V.; Gerber, M.; Boutron-Ruault, M.-C.; Joulin, V.; Lenoir, G.; Berrino, F.; Riboli, E.; Benichou, J.; Clavel-Chapelon, F. Dietary intakes of x-6 and x-3 polyunsaturated fatty acids and the risk of breast cancer. Int. J. Cancer 2009, 124, 924–931.
- El-Bahr, S.M.; Shousha, S.; Alfattah, M.A.; Al-Sultan, S.; Khattab, W.; Sabeq, I.I.; Ahmed-Farid, O.; El-Garhy, O.; Albusadah, K.A.; Alhojaily, S.; et al. Enrichment of Broiler Chickens’ Meat with Dietary Linseed Oil and Lysine Mixtures: Influence on Nutritional Value, Carcass Characteristics and Oxidative Stress Biomarkers. Foods 2021, 10, 618.
- Konieczka, P.; Czauderna, M.; Smulikowska, S. The enrichment of chicken meat with omega-3 fatty acids by dietary fish oil or its mixture with rapeseed or flaxseed—Effect of feeding duration Dietary fish oil, flaxseed, and rapeseed and n-3 enriched broiler meat. Anim. Feed Sci. Technol. 2017, 223, 42–52.
- Aziza, A.E.; Quezada, N.; Cherian, G. Feeding Camelina sativa meal to meat-type chickens: Effect on production performance and tissue fatty acid composition. J. App. Poult. Res. 2010, 19, 157–168.
- Ryhänen, E.-L.; Pertilä, S.; Tupasela, T.; Valaja, J.; Eriksson, C.; Larkka, K. Effect of Camelina sativa expeller cake on performance and meat quality of broilers. J. Sci. Food Agric. 2007, 87, 1489–1494.
- Lolli, S.; Grilli, G.; Ferrari, L.; Battelli, G.; Pozzo, S.; Galasso, I.; Russo, R.; Brasca, M.; Reggiani, R.; Ferrante, V. Effect of Different Percentage of Camelina sativa Cake in Laying Hens Diet: Performance, Welfare, and Eggshell Quality. Animals 2020, 10, 1396.
- Dӧnmez, E.O.; Belli, O. Urartian plant cultivation at Yoncatepe (Van), eastern Turkey. Econ. Bot. 2007, 61, 290–298.
- Hovsepyan, R.; Willcox, G. The earliest finds of cultivated plants in Armenia: Evidence from charred remains and crop processing residues in pisé from the Neolithic settlements of Aratashen and Aknashen. Veg. Hist. Archaeobot. 2008, 17 (Suppl. 1), 63–71.
- Kroll, H. Agriculture and arboriculture in mainland Greece at the beginning of the first millenium B.C. Pallas 2000, 52, 61–68.
- Van Zeist, W.A. Plant remains from Iron Age Noordbarge, province of Drenthe, the Netherlands. Palaeohistoria 1981, 23, 169–193.
- Matthäus, B.; Zubr, J. Variability of specific components in Camelina sativa oilseed cakes. Ind. Crops Prod. 2000, 12, 9–18.
- Mohammad, B.T.; Al-Shannag, M.; Alnaief, M.; Singh, L.; Singsaas, E.; Alkasrawi, M. Production of multiple biofuels from whole camelina material: A renewable energy crop. BioResources 2018, 13, 4870–4883.
- Neupane, D.; Solomon, J.K.Q.; Mclennon, E.; Davison, J.; Lawry, T. Sowing date and sowing method influence on camelina cultivars grain yield, oil concentration, and biodiesel production. Food Energy Secur. 2019, 8, e00166.
- Yang, J.; Caldwell, C.; Corscadden, K.; He, Q.; Li, J. An evaluation of biodiesel production from Camelina sativa grown in Nova Scotia. Ind. Crops Prod. 2016, 81, 162–168.
- Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of Feed Materials. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013R0068&from=EN (accessed on 21 September 2021).
- Ciurescu, G.; Ropota, M.; Toncea, I.; Habeanu, M. Camelia (Camelia sativa L. Crantz Variety) Oil and Seeds as n-3 Fatty Acids Rich Products in Broiler Diets and Its Effects on Performance, Meat Fatty Acid Composition, Immune Tissue Weights, and Plasma Metabolic Profile. J. Agr. Sci. Tech. 2016, 18, 315–326.
- Hilbrands, A.M.; Johnston, L.J.; Cox, R.B.; Forcella, F.; Gesch, R.; Li, Y.Z. Effects of increasing dietary inclusion of camelina cake on growth performance of growing-finishing pigs. Transl. Anim. Sci. 2021, 5, 1–10.
- Pekel, A.Y.; Kim, J.L.; Chapple, C.; Adeola, O. Nutritional characteristics of camelina meal for 3 week-old broiler chickens. Poult. Sci. 2015, 94, 371–378.
- Bulbul, T.; Rahmann, A.; Ozdemir, V. Effect of False Flax Meal on Certain Growth Serum and Meat Parameters of Japanese Quails. J. Anim. Plant Sci. 2015, 25, 1245–1250.
- Oryschak, M.A.; Christianson, C.B.; Beltranena, E. Camelina sativa cake for broiler chickens: Effects of increasing dietary inclusion on clinical signs of toxicity, feed disappearance, and nutrient digestibility. Transl. Anim. Sci. 2020, 4, 1263–1277.
- Woyengo, T.A.; Beltranena, E.; Zijlstra, R.T. Effect of anti-nutritional factors of oilseed co-product on feed intake of pigs and poultry. Anim. Feed Sci. Technol. 2017, 233, 76–86.
- Jaśkiewicz, T.; Sagan, A.; Puzio, I. Effect of the Camelina sativa oil on the performance, essential fatty acid level in tissues and fat—Soluble vitamins content in the livers of broiler chickens. Livest. Sci. 2014, 165, 74–79.
- Pilgeram, A.L.; Sands, D.S.; Boss, D.; Dale, N.; Wichman, D.; Lamb, P.; Lu, C.; Barrows, R.; Kirkpatrick, M.; Thompson, B.; et al. Camelina sativa, a Montana omega-3 and fuel crop. In Issues in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, Egypt, 2007; pp. 129–131.
- Banaszkiewicz, T. Nutritional Value of Soybean Meal. In Soybean and Nutrition; El-Shemy, H., Ed.; IntechOpen: 2011; pp. 1–20. Available online: https://www.intechopen.com/books/soybean-and-nutrition/nutritional-value-of-soybean-meal (accessed on 18 May 2021).
- Daszykowski, M.; Wrobel, M.S.; Czarnik-Matusewicz, H.; Walczak, B. Near-infrared reflectance spectroscopy and multivariate calibration techniques applied to modelling the crude protein, fiber and fat content in rapeseed meal. Analyst 2008, 133, 1523–1531.
- Feng, D.; Zuo, J. Nutritional and anti-nutritional composition of rapeseed meal and its utilization as a feed ingredient for animal. In Feed and Industrial Raw Material: Feed. Available online: https://www.gcirc.org/fileadmin/documents/Proceedings/IRCWuhan2007%20vol5/Pages_de_vol-5-37.pdf (accessed on 21 September 2021).
- Kaczmarek, P.; Korniewicz, D.; Lipiński, K.; Mazur, M. Chemical Composition of Rapeseed Products and their use in Pig Nutrition. Polish J. Nat. Sci. 2016, 31, 545–562.
- Thacker, P.; Widyaratne, G. Effects of expeller pressed camelina meal and/or canola meal on digestibility, performance and fatty acid composition of broiler chickens fed wheat-soybean meal-based diets. Arch. Anim. Nutr. 2012, 66, 402–415.
- Almeida, F.N.; Htoo, J.K.; Thompson, J.; Stein, H.H. Amino acid digestibility in camelina products fed to growing pigs. Can. J. Anim. Sci. 2013, 93, 335–343.
- Canola Council of Canada. 2015. Canola Meal Feeding Guide. Feed Industry Guide. Available online: https://www.canolacouncil.org/media/516716/2015_canola_meal_feed_industry_guide.pdf (accessed on 18 May 2020).
- Chen, C.C.; Shih, Y.C.; Chiou, P.W.S.; Yu, B. Evaluating Nutritional Quality of Single Stage—And Two Stage-fermented Soybean Meal. Asian-Aus. J. Anim. Sci. 2010, 23, 598–606.
- Stein, H.H. Amino acid digestibility in four sources of canola meal and soybean meal fed to growing pigs. Available online: https://nutrition.ansci.illinois.edu/node/653 (accessed on 18 May 2020).
- Narducci, V.; Finotti, E.; Galli, V.; Carcea, M. Lipids and Fatty Acids in Italian Durum Wheat (Triticum durum Desf.) Cultivars. Foods 2019, 8, 223.
- Opapeju, F.O.; Nyachoti, C.M.; House, J.D.; Weiler, H.; Sapirstein, H.D. Growth performance and carcass characteristics of pigs fed short-season corn hybrids. J. Anim. Sci. 2006, 84, 2779–2786.
- Akkaya, M.R. Fatty acid compositions of sunflowers (Helianthus annuus L.) grown in east Mediterranean region. Riv. Ital. Delle Sostanze Grasse 2018, XCV, 239–247.
- Anca, G.; Habeanu, M.; Lefter, N.A.; Ropota, M. Performance Parameters Plasma Lipid Status, and Lymphoid Tissue Fatty Acid Profile of Broiler Chicks Fed Camelina Cake. Rev. Bras. Cienc. Avic. 2019, 21, 001–008.
- Bailoni, L.; Bortolozzo, A.; Mantovani, R.; Simonetto, A.; Schiavon, S.; Bittante, G. Feeding dairy cows with full fat extruded or toasted soybean seeds as replacement of soybean meal and effects on milk yield, fatty acid profile and CLA content. Ital. J. Anim. Sci. 2004, 3, 243–258.
- Juodka, R.; Juska, R.; Juskiene, V.; Leikus, R.; Stankeviciene, D.; Nainiene, R. The effect of feeding with hemp and Camelina cakes on the fatty acid profile of duck muscles. Arch. Anim. Breed. 2018, 61, 293–303.
- Abu-Ghazaleh, A.A.; Schingoethe, D.J.; Hippen, A.R. Conjugated Linoleic Acid and Other Beneficial Fatty Acids in Milk Fat from Cows Fed Soybean Meal, Fish Meal, or Both. J. Dairy Sci. 2001, 84, 1845–1850.
- Abramovič, H.; Abram, V. Physico-Chemical Properties, Composition and Oxidative Stability of Camelina sativa Oil. Food Technol. Biotechnol. 2005, 43, 63–70.
- Baltrukoniene, G.; Uchockis, V.; Švirmickas, G.J. The influence of compound feed enrichment with rapeseed and linseed cake on the meat characteristics and fatty acids composition of beef bulls. Zemdirbyste-Agriculture 2015, 102, 319–324.
- Halle, I.; Schöne, F. Influence of rapeseed cake, linseed cake and hemp seed cake on laying performance of hens and fatty acid composition of egg yolk. JCF 2013, 8, 185–193.
- Lee, J.W.; Kil, D.Y.; Keever, B.D.; Killefer, J.; McKeith, F.K.; Sulabo, R.C.; Stein, H.H. Carcass fat quality of pigs is not improved by adding corn germ, beef tallow, palm kernel oil, or glycerol to finishing diets containing distillers dried grains with solubles. J. Anim. Sci. 2013, 91, 2426–2437.
- Mierlita, D. Effects of diets containing hemp seeds or hemp cake on fatty acid composition and oxidative stability of sheep milk. S. Afr. J. Anim. Sci. 2018, 48, 504–515.
- Abramovič, H.; Butinar, B.; Nikolič, V. Changes occurring in phenolic content, tocopherol composition and oxidative stability of Camelina sativa oil during storage. Food Chem. 2007, 104, 903–909.
- Zubr, J.; Matthaus, B. Effects of growth conditions on fatty acids and tocopherols in Camelina sativa oil. Ind. Crops Prod. 2002, 15, 155–162.
- Kurasiak-Popowska, D.; Ryńska, B.; Stuper-Szablewska, K. Analysis of Distribution of Selected Bioactive Compounds in Camelina sativa from Seeds to Pomace and Oil. Agronomy 2019, 9, 168.
- Kurasiak-Popowska, D.; Stuper-Szablewska, K. The phytochemical quality of Camelina sativa seed and oil. Acta Agric Scand B Soil Plant Sci 2020, 70, 39–47.
- Schuster, A.; Friedt, W. Glucosinolate content and composition as parameters of quality of camelina seed. Ind. Crops Prod. 1998, 7, 297–302.
- Russo, R.; Reggiani, R. Antinutritive Compounds in Twelve Camelina sativa Genotypes. Am. J. Plant Sci. 2012, 3, 1408–1412.
- Colombini, S.; Broderick, G.A.; Galasso, I.; Martinelli, T.; Rapetti, L.; Russo, R.; Reggiani, R. Evaluation of Camelina sativa (L.) Meal as an Alternative Protein Source in Ruminant Rations. J. Sci. Food Agric. 2014, 94, 736–743.
- Russo, R.; Reggiani, R. Glucosinolates and Sinapine in camelina meal. Food Sci. Nutr. 2017, 8, 1063–1073.
- Yuan, D.; Shim, Y.Y.; Shen, J.; Jadhav, P.D.; Meda, V.; Reaney, M.J.T. Distribution of glucosinolates in camelina seed fractions by HPLC-ESI-MS/MS. Eur. J. Lipid Sci. Technol. 2017, 119, 1600040.
- Tripathi, M.K.; Mishra, A.S. Glucosinolates in animal nutrition: A review. Anim. Feed Sci. Technol. 2007, 132, 1–27.
- Matthäus, B.; Angelini, L.G. Anti-Nutritive Constituents in Oilseed Crops from Italy. Ind. Crops Prod. 2005, 21, 89–99.
- Go, Y. Lineage-specific expansions and contractions of the bitter taste receptor gene repertoire in vertebrates. Mol. Biol. Evol. 2006, 23, 964–972.
- Qiao, H.; Classen, H.L. Nutritional and physiological effects of rapeseed meal sinapine in broiler chickens and its metabolism in the digestive tract. J. Sci. Food Agric. 2003, 83, 1430–1438.
- Matthäus, B. Antinutritive compounds in different oilseeds. Lipid/Fett 1997, 99, 170–174.
- Singleton, L. Naturally Occurring Food Toxicants: Phenolic Substances of Plant Origin Common in Foods. Adv. Food Res. 1981, 27, 149–242.
- Amarowicz, R.; Estrella, I.; Hernández, T.; Robredo, S.; Troszynska, A.; Kosinska, A.; Pegg, R.B. Free Radical-Scavenging Capacity, Antioxidant Activity and Phenolic Composition of Green Lentil (Lens culinaris). Food Chem. 2010, 121, 705–711.
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the European Commission on glucosinolates as undesirable substances in animal feed. EFSA J. 2008, 590, 1–76.
- Schill, S.R. 2009. Camelina Meal Approved for Feedlot Cattle. Biodiesel Magazine 2009. Available online: http://www.biodieselmagazine.com/articles/3837/camelinameal-approved-for-feedlot-cattle (accessed on 20 October 2020).
- Orczewska-Dudek, S.; Pietras, M. The Effect of Dietary Camelina sativa Oil or Cake in the Diets of Broiler Chickens on Growth Performance, Fatty Acid Profile, and Sensory Quality of Meat. Animals 2019, 9, 734.
- Frame, D.D.; Palmer, M.; Peterson, B. Use of Camelina sativa in the Diets of Young Turkeys. J. Appl. Poult. Res. 2007, 16, 381–386.
- Ciurescu, G.; Hebean, V.; Tamaş, V.; Burcea, D. Use of Dietary Camelina (Camelina sativa) Seeds During the Finishing Period: Effects on Broiler Performance and on the Organoleptic Traits of Broiler Meat. J. Anim. Sci. Biotechnol. 2007, 40, 410–417.
- Pietras, M.P.; Orczewska-Dudek, S. The effect of dietary Camelina Sativa oil on quality of broiler chicken meat. Ann. Anim. Sci. 2013, 13, 869–882.
- Pekel, A.Y.; Patterson, P.H.; Hulet, R.M.; Acar, N.; Cravener, T.L.; Dowler, D.B.; Hunter, J.M. Dietary camelina meal versus flaxseed with and without supplemental copper for broiler chickens: Live performance and processing yield. Poult. Sci. 2009, 88, 2392–2398.
- Aziza, A.; Awadin, W.F.; Quezada, N.; Cherian, G. Gastrointestinal morphology, fatty acid profile, and production performance of broiler chicken fed camelina meal or fish oil. Eur. J. Lipid Sci. Technol. 2014, 116, 1727–1733.
- Burel, C.; Boujard, T.; Kaushik, S.J.; Boeuf, G.; Mol, K.A.; Van der Geyten, S.; Darras, V.M.; Kühn, E.R.; Pradet-Balade, B.; Querat, B.; et al. Effects of rapeseed meal-glucosinolates on thyroid metabolism and feed utilization in rainbow trout. Gen. Comp. Endocrinol. 2001, 124, 343–358.
- Martínez, Y.; Valdivié, M. Efficiency of Ross 308 broilers under different nutritional requirements. J. Appl. Poult. Res. 2021, 30, 100140.
- Slominski, B.A. Recent advances in research on enzymes for poultry diets. Poult. Sci. 2011, 90, 2013–2023.
- Woyengo, T.A.; Patterson, R.; Slominski, B.A.; Beltranena, E.; Zijlstra, R.T. Nutritive value of cold-pressed camelina cake with or without supplementation of multi-enzyme in broiler chickens. Poult. Sci. 2016, 95, 2314–2321.
- Del Puerto, M.; Cabrera, M.C.; Saadoun, A. A Note on Fatty Acids Profile of Meat from Broiler Chickens Supplemented with Inorganic or Organic Selenium. Int. J. Food Sci. 2017, 7613069.
- Crespo, N.; Esteve-Garcia, E. Dietary Fatty Acid Profile Modifies Abdominal Fat Deposition in Broiler Chickens. Poult. Sci. 2001, 80, 71–78.
- Trembecka, L.; Haščik, P.; Čubon, J.; Bobko, M.; Pavelkova, A. Fatty acids profile of breast and thigh muscles of broiler chickens fed diets with propolis and probiotics. J. Cent. Eur. Agric. 2016, 17, 1179–1193.
- Zdunczyk, Z.; Gruzauskas, R.; Juskiewicz, J.; Semaskaite, A.; Jankowski, J.; Godycka-Klos, I.; Jarule, V.; Miezeliene, A.; Alencikiene, G. Growth performance, gastrointestinal tract responses, and meat characteristics of broiler chickens fed a diet containing the natural alkaloid sanguinarine from Macleaya cordata. J. Appl. Poult. Res. 2010, 19, 393–400.
- Khatibjoo, A.; Kermanshahi, H.; Golian, A.; Zaghari, M. The effect of n-6/n-3 fatty acid ratios on broiler breeder performance, hatchability, fatty acid profile and reproduction. J. Anim. Physiol. Anim. Nutr. 2018, 102, 986–998.
- Nguyen, L.Q.; Nuijens, N.C.G.A.; Everts, H.; Salden, H.; Beynen, A.C. Mathematical relationships between the intake of n-6 and n-3 polyunsaturated fatty acids and their contents in adipose tissue of growing pig. Meat Sci. 2003, 65, 1399–1406.
- Kanakri, K.; Carragher, J.; Hughes, R.; Muhlhausler, B.; Gibson, R. The Effect of Different Dietary Fats on the Fatty Acid Composition of Several Tissues in Broiler. Eur. J. Lipid Sci. Tech. 2018, 120, 1700237.
- Nain, S.; Oryschak, M.A.; Betti, M.; Beltranena, E. Camelina sativa cake for broilers: Effects of increasing dietary inclusion from 0 to 24% on tissue fatty acid proportions at 14, 28, and 42 d of age. Poult. Sci. 2015, 94, 1247–1258.
- Aronen, I.; Valkonen, E.; Tupasela, T.; Hiidenhovi, J.; Valaja, J. The Effect of Camelina Sativa Cake on Fatty Acid Composition and Sensory Quality of Eggs and Broiler Meat. 2009. Available online: https://pdfs.semanticscholar.org/748c/be17aeb67241466db54687c3dc5d96642336.pdf?_ga=2.253177551.710654730.1589791031-675995229.1575974901 (accessed on 25 November 2021).
- Chen, X.; Du, X.; Shen, J.; Lu, L.; Wang, W. Effect of various dietary fats on fatty acid profile in duck liver: Efficient conversion of short-chain to long-chain omega-3 fatty acids. Exp. Biol. Med. 2017, 242, 80–87.
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is docosahexaenoic acid synthesis from-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66.
- Rymer, C.; Givens, D.I. Effect of species and genotype on the efficiency of enrichment of poultry meat with n-3 polyunsaturated fatty acids. Lipids 2006, 41, 445–451.
- Gregory, M.K.; Geier, M.S.; Gibson, R.A.; James, M.J. Functional Characterization of the Chicken Fatty Acid Elongases. J. Nutr. 2013, 143, 12–16.
- Jing, M.; Zhao, S.; House, J.D. Performance and tissue fatty acid profile of broiler chickens and laying hens fed hemp oil and HempOmegaTM. Poult. Sci. 2017, 96, 1809–1819.
- Gonzalez-Esquerra, R.; Leeson, S. Alternatives for enrichment of eggs and chicken meat with n-3 fatty acids. Can. J. Anim. Sci. 2001, 81, 295–305.
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358.
- Wang, Y.; Botolin, D.; Christian, B.; Busik, J.; Xu, J.; Jump, D.B. Tissue specific, nutritional, and developmental regulation of rat fatty acid elongases. J. Lipid Res. 2005, 46, 706–715.
- Jing, M.; Gakhar, N.; Gibson, R.A.; House, J.D. Dietary and ontogenic regulation of fatty acid desaturase and elongase expression in broiler chickens. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 107–113.
- Gou, Z.Y.; Cui, X.Y.; Li, L.; Fan, Q.L.; Lin, X.J.; Wang, Y.B.; Jiang, Z.Y.; Jiang, S.Q. Effects of dietary incorporation of linseed oil with soybean isoflavone on fatty acid profiles and lipid metabolism-related gene expression in breast muscle of chickens. Animal 2020, 14, 2414–2422.
- Okrouhla, M.; Stupka, R.; Cítek, J.; Šprysl, M.; Brzobohatý, L. Effect of dietary linseed supplementation on the performance, meat quality, and fatty acid profile of pigs. Czech J. Anim. Sci. 2013, 58, 279–288.
- European Commission Nutrition Claims. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/nutrition_claims_en (accessed on 16 October 2021).
