Khat (Catha edulis) is a recreational, chewed herbal drug that has been used as a psychostimulant for centuries in East Africa and the Arabian Peninsula, namely in Somalia, Ethiopia, and Yemen. However, the growing worldwide availability of khat has produced widespread concern. The plant comprises a large number of active substances, among which cathinone, cathine, and norephedrine are the main constituents, which can be included in the group of sympathomimetics of natural origin. In fact, these compounds are amphetamine analogues, and, as such, they have amphetamine-like nervous system stimulant effects. Chewing the leaves gives people a sensation of well-being and increases energy, alertness, and self-confidence. The chronic use of khat is, however, associated with severe cardiac, neurological, psychological, and gastrointestinal complications. The psychological dependence and withdrawal symptoms of khat are the reasons for its prolonged use.
- cathinone
- cathine
- kinetics
- toxicology
- amphetamine-like
- norpseudoephedrine
1. Introduction
2. Khat Phytochemistry
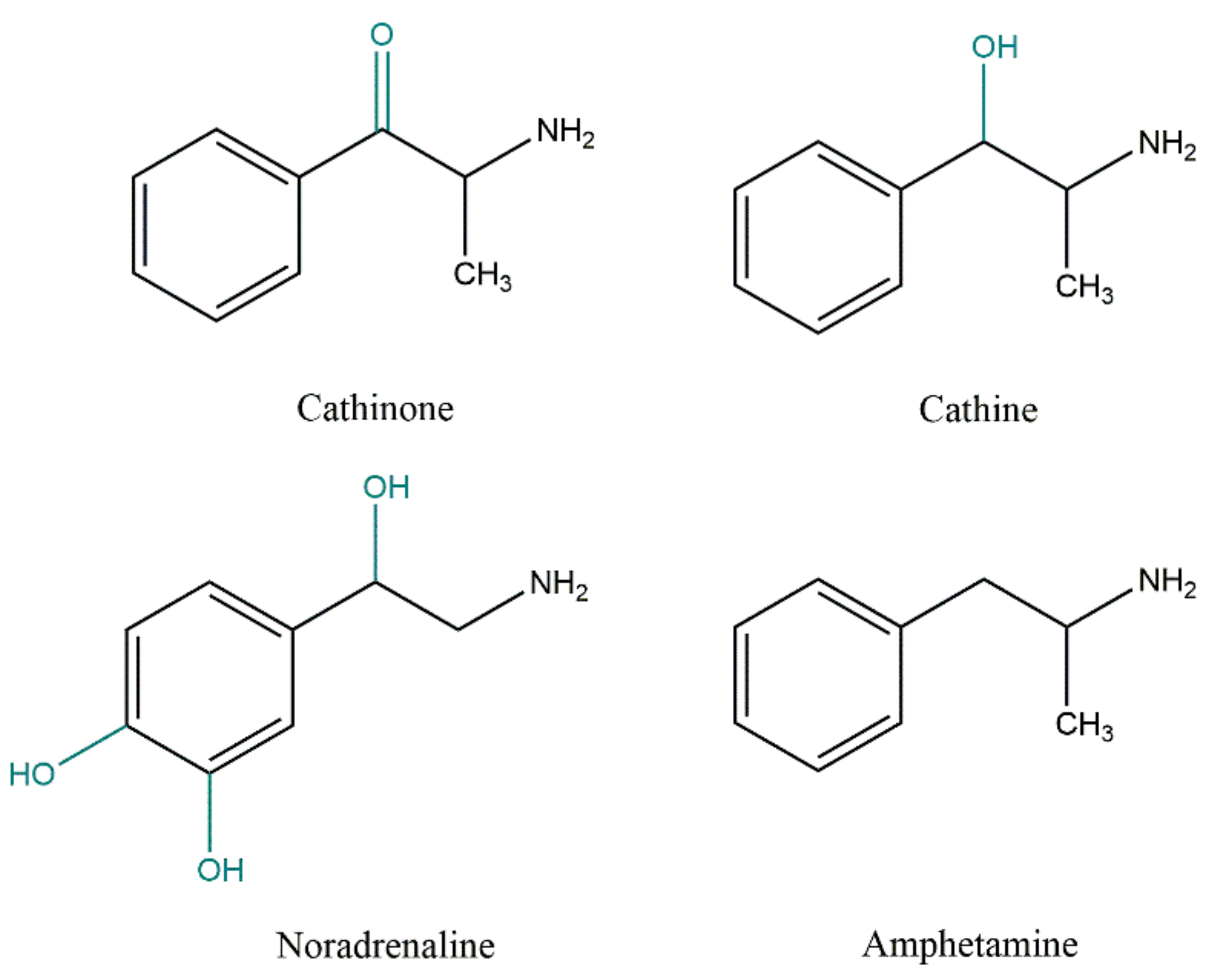
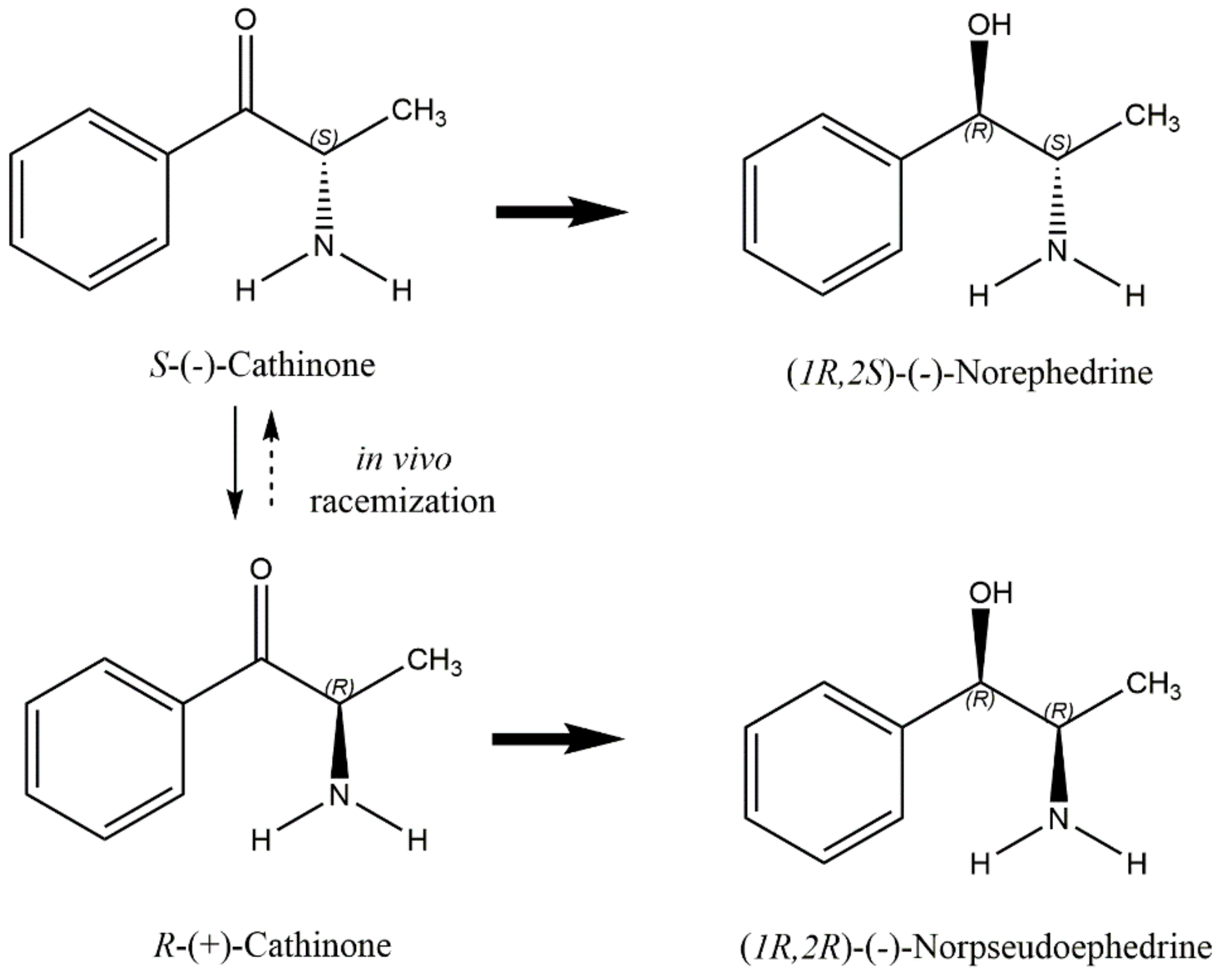
3. Khat Toxicokinetics
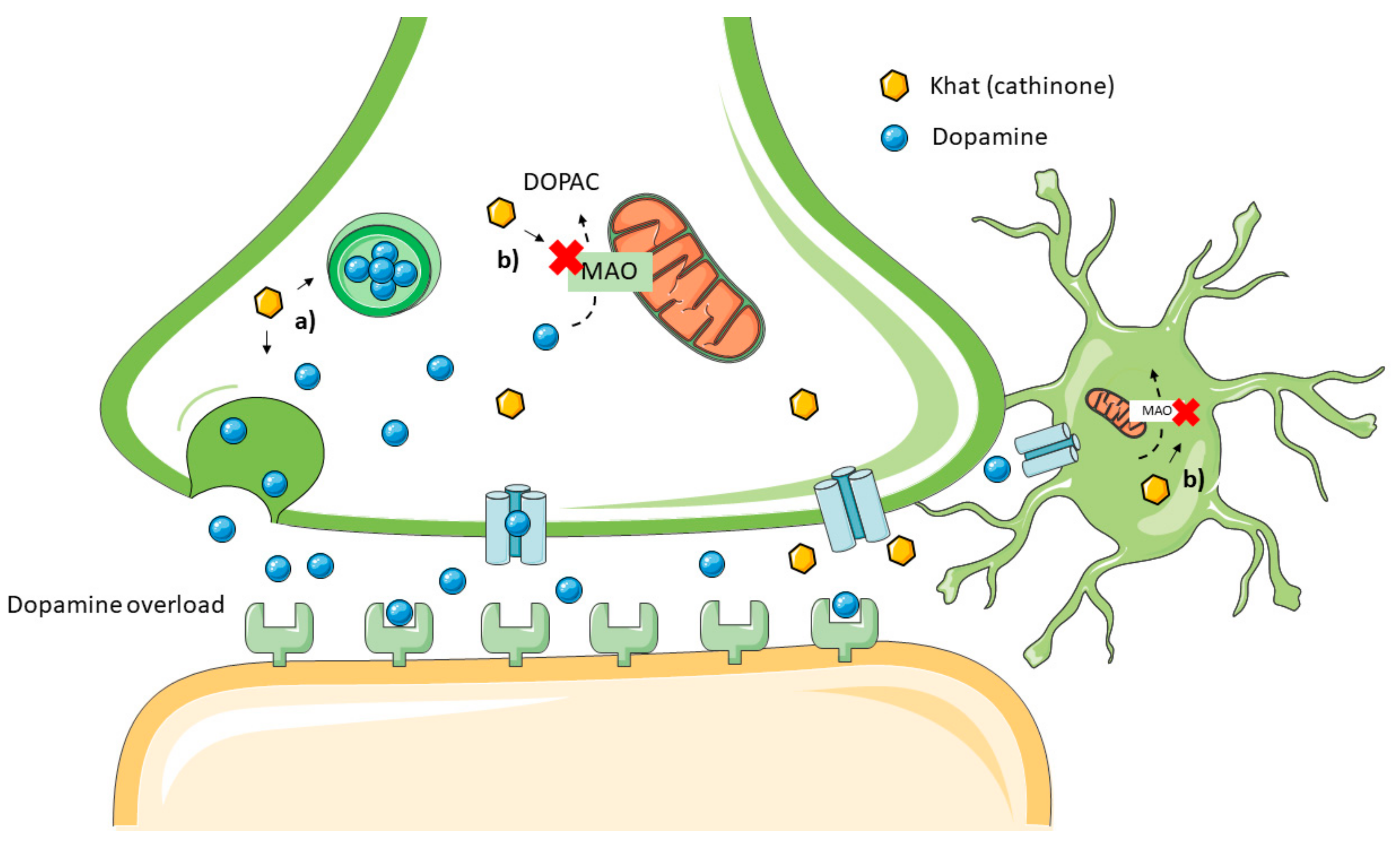
4. Khat Toxicodynamic
5. Addiction
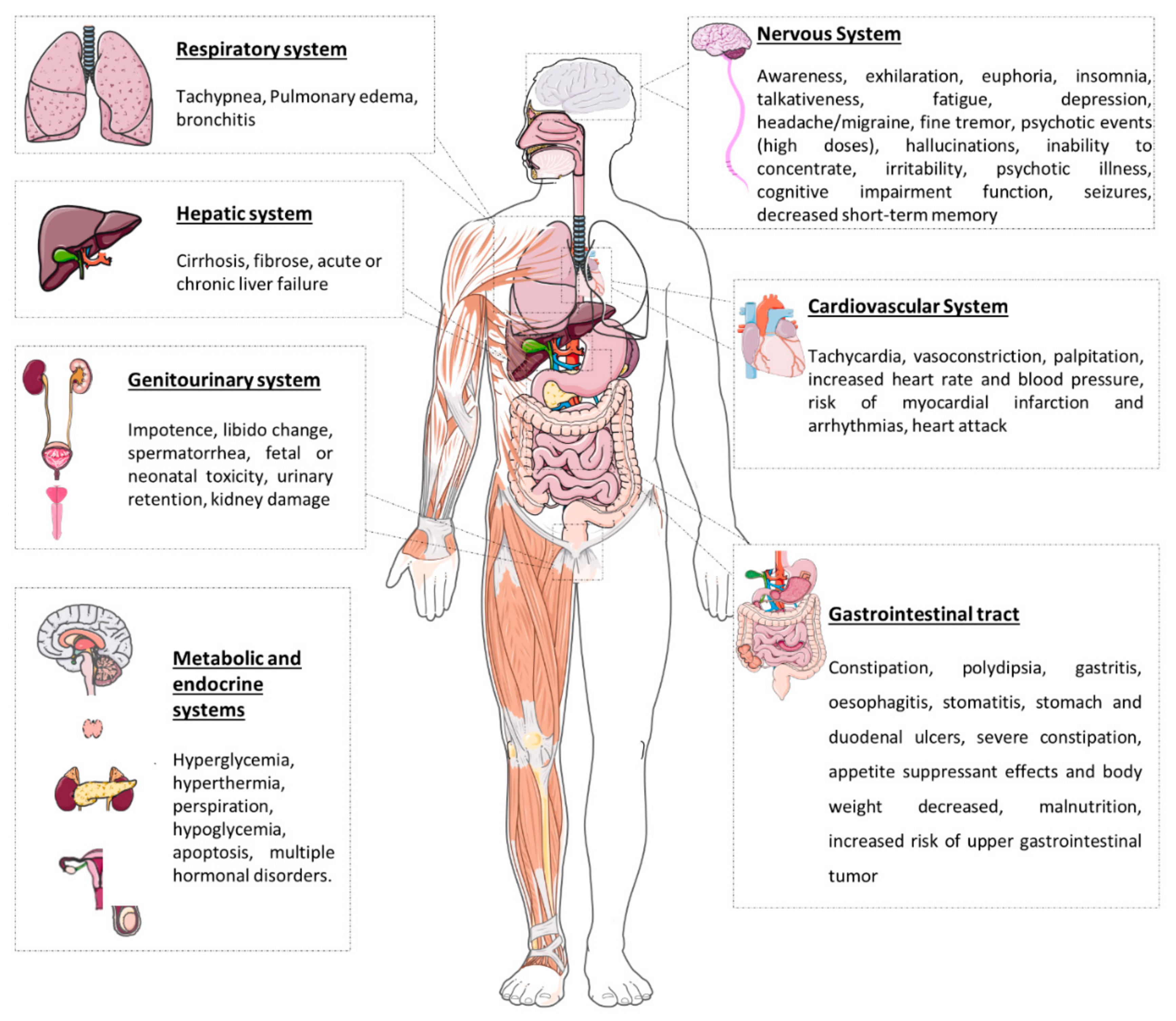
6. Effects after Chewing Khat Leaves
6.1. In Vitro Studies
Table 1 recaps all the in vitro studies.
Table 1. In vitro studies of khat.
|
In Vitro Model |
Concentration ofKhat Extract |
Results |
|
0–80 ng/mL |
LD50 of 40 ng/mL DNA synthesis inhibition by 50% at 200 ng/mL |
|
|
Biphasic survival (LD50 of 20 ng/mL for 25% of the cell population and 75 ng/mL for the more resistant subpopulation) DNA synthesis inhibition by 50% at 45 ng/mL in 1BR.3 cells, and 60 ng/mL in XP2Bi cells |
||
|
0.06, 0.6, 6, 30 and 60 mg/mL |
The highest concentrations (30 mg/mL and 60 mg/mL): significantly inhibited testosterone production and decreased the cell viability The lowest concentrations (0.06, 0.6, and 6 mg/mL): significantly stimulated testosterone production and had no effect on interstitial viability |
|
|
HL-60, Jurkat, NB4 cell lines, and |
Organic khat extract induced apoptotic cell death, regulated by the activation of cellular caspase −1, −3, and −8 |
|
|
200 μg/mL |
Organic khat extract activated a distinct cell death involving mitochondrial damage and morphological features of autophagy |
|
|
0.01, 0.03, 0.1, 0.3, 1, 3, and 10 mg/mL |
Khat induces reduced cell size, cell membrane damage, and apoptosis The highest concentrations (1, 3, and 10 mg/mL) affected cell metabolic activity, cell cycle, and cellular proliferation |
|
|
Khat constituents (cathine, cathinone, and catheduline): bound to family A of G-protein-coupled receptors and altered several signalling pathways (CREB, Wnt, FGF, IL-6, and ERK/MAPK) |
6.2. Human Studies
References
- Abebe, W. Khat: A Substance of Growing Abuse with Adverse Drug Interaction Risks. J. Natl. Med Assoc. 2018, 110, 624–634.
- Balint, E.E.; Falkay, G.; Balint, G.A. Khat—A controversial plant. Wien Klin Wochenschr 2009, 121, 604–614.
- Valente, M.J.; de Pinho, P.G.; Bastos, M.D.L.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45.
- Al-Hebshi, N.N.; Skaug, N. Khat (Catha edulis)—An updated review. Addict. Biol. 2005, 10, 299–307.
- Al-Motarreb, A.; Baker, K.; Broadley, K.J. Khat: Pharmacological and medical aspects and its social use in Yemen. Phytother. Res. 2002, 16, 403–413.
- Szendrei, K. The chemistry of khat. Bull. Narc. 1980, 32, 5–35.
- Kalix, P. Cathinone, a natural amphetamine. Pharmacol. Toxicol. 1992, 70, 77–86.
- Kelly, J.P. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test. Anal. 2011, 3, 439–453.
- Brenneisen, R.; Geisshüsler, S. Psychotropic drugs.III. Analytical and chemical aspects of Catha edulis Forsk. Pharm. Acta Helv. 1985, 60, 290–301.
- Kalix, P.; Braenden, O. Pharmacological aspects of the chewing of khat leaves. Pharmacol. Rev. 1985, 37, 149–164.
- Geisshüsler, S.; Brenneisen, R. The content of psychoactive phenylpropyl and phenylpentenyl khatamines in Catha edulis Forsk. of different origin. J. Ethnopharmacol. 1987, 19, 269–277.
- Kalix, P. Pharmacological properties of the stimulant khat. Pharmacol. Ther. 1990, 48, 397–416.
- Feyissa, A.M.; Kelly, J.P. A review of the neuropharmacological properties of khat. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1147–1166.
- Feng, L.-Y.; Battulga, A.; Han, E.; Chung, H.; Li, J.-H. New psychoactive substances of natural origin: A brief review. J. Food Drug Anal. 2017, 25, 461–471.
- Patel, N.B. Khat (Catha edulis Forsk)—And now there are three. Brain Res. Bull. 2019, 145, 92–96.
- Sawair, F.A.; Al-Mutwakel, A.; Al-Eryani, K.; Al-Surhy, A.; Maruyama, S.; Cheng, J.; Al-Sharabi, A.; Saku, T. High relative frequency of oral squamous cell carcinoma in Yemen: Qat and tobacco chewing as its aetiological background. Int. J. Environ. Health Res. 2007, 17, 185–195.
- Toennes, S.W.; Harder, S.; Schramm, M.; Niess, C.; Kauert, G.F. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. Br. J. Clin. Pharmacol. 2003, 56, 125–130.
- Toennes, S.W.; Kauert, G.F. Excretion and detection of cathinone, cathine, and phenylpropanolamine in urine after kath chewing. Clin. Chem. 2002, 48, 1715–1719.
- Brenneisen, R.; Geisshüsler, S.; Schorno, X. Metabolism of cathinone to (−)-norephedrine and (−)-norpseudoephedrine. J. Pharm. Pharmacol. 1986, 38, 298–300.
- Mathys, K.; Brenneisen, R. Determination of (S)-(−)-cathinone and its metabolites (R,S)-(−)-norephedrine and (R,R)-(−)-norpseudoephedrine in urine by high-performance liquid chromatography with photodiode-array detection. J. Chromatogr. 1992, 593, 79–85.
- Engidawork, E. Pharmacological and Toxicological Effects of Catha edulisF. (Khat). Phytotherapy Res. 2017, 31, 1019–1028.
- Odenwald, M.; al’Absi, M. Khat use and related addiction, mental health and physical disorders: The need to address a growing risk. East Mediterr. Health J. 2017, 23, 236–244.
- Cox, G.; Rampes, H. Adverse effects of khat: A review. Adv. Psychiatr. Treat. 2003, 9, 456–463.
- Heal, D.J.; Smith, S.L.; Gosden, J.; Nutt, D.J. Amphetamine, Past and present—A pharmacological and clinical perspective. J. Psychopharmacol. 2013, 27, 479–496.
- Mladěnka, P.; Applová, L.; Patočka, J.; Costa, V.M.; Remiao, F.; Pourová, J.; Mladěnka, A.; Karlíčková, J.; Jahodář, L.; Vopršalová, M.; et al. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med Res Rev 2018, 38, 1332–1403.
- Nencinp, P.; Amiconi, G.; Befani, O.; Abdullahi, M.; Anania, M. Possible involvement of amine oxidase inhibition in the sympathetic activation induced by khat (catha edulis) chewing in humans. J. Ethnopharmacol. 1984, 11, 79–86.
- Osorio-Olivares, M.; Rezende, M.C.; Sepúlveda-Boza, S.; Cassels, B.K.; Fierro, A. MAO inhibition by arylisopropylamines: The effect of oxygen substituents at the beta-position. Bioorg. Med. Chem. 2004, 12, 4055–4066.
- Basker, G. A review on hazards of khat chewing. Int. J. Pharm. Pharm. Sci. 2013, 5, 74–77.
- Nielen, R.J.; A Van Der Heijden, F.M.M.; Tuinier, S.; A Verhoeven, W.M. Khat and mushrooms associated with psychosis. World J. Biol. Psychiatry 2004, 5, 49–53.
- Patel, N.B. Mechanism of action of cathinone: The active ingredient of khat (Catha edulis). East Afr. Med J. 2009, 77, 329–332.
- Al-Ahdal, M.N.; McGarry, T.J.; Hannan, M.A. Cytotoxicity of Khat (Catha edulis) extract on cultured mammalian cells: Effects on macromolecule biosynthesis. Mutat. Res. 1988, 204, 317–322.
- Nyongesa, A.W.; Patel, N.B.; Onyango, D.W.; Wango, E.O.; Odongo, H.O. In vitro study of the effects of khat (Catha edulis Forsk) extract on isolated mouse interstitial cells. J. Ethnopharmacol. 2007, 110, 401–405.
- Dimba, E.A.; Gjertsen, B.T.; Bredholt, T.; Fossan, K.O.; Costea, D.E.; Francis, G.W.; Johannessen, A.C.; Vintermyr, O.K. Khat (Catha edulis)-induced apoptosis is inhibited by antagonists of caspase-1 and -8 in human leukaemia cells. Br. J. Cancer 2004, 91, 1726–1734.
- Bredholt, T.; Dimba, E.A.; Hagland, H.R.; Wergeland, L.; Skavland, J.; O Fossan, K.; Tronstad, K.J.; Johannessen, A.C.; Vintermyr, O.K.; Gjertsen, B.T. Camptothecin and khat (Catha edulis Forsk.) induced distinct cell death phenotypes involving modulation of c-FLIPL, Mcl-1, procaspase-8 and mitochondrial function in acute myeloid leukemia cell lines. Mol. Cancer 2009, 8, 101.
- Abou-Elhamd, A.S.; Kalamegam, G.; Ahmed, F.; Assidi, M.; Alrefaei, A.F.; Pushparaj, P.N.; Abu-Elmagd, M. Unraveling the Catha edulis Extract Effects on the Cellular and Molecular Signaling in SKOV3 Cells. Front. Pharmacol. 2021, 12, 666885.
- Abebe, M.; Kindie, S.; Adane, K. Adverse Health Effects of Khat: A Review. Fam. Med. Med. Sci. Res. 2015, 4, 154.
- Widler, P.; Mathys, K.; Brenneisen, R.; Kalix, P.; Fisch, H.-U. Pharmacodynamics and pharmacokinetics of khat: A controlled study. Clin. Pharmacol. Ther. 1994, 55, 556–562.
- Hill, C.M.; Gibson, A. The oral and dental effects of q’at chewing. Oral. Surg. Oral. Med. Oral. Pathol. 1987, 63, 433–436.
- Goldenberg, D.; Lee, J.; Koch, W.M.; Kim, M.M.; Trink, B.; Sidransky, D.; Moon, C.-S. Habitual Risk Factors for Head and Neck Cancer. Otolaryngol. Neck Surg. 2004, 131, 986–993.
- Al-Habori, M. The potential adverse effects of habitual use of Catha edulis(khat). Expert Opin. Drug Saf. 2005, 4, 1145–1154.
- Heymann, T.D.; Bhupulan, A.; Zureikat, N.E.K.; Bomanji, J.; Drinkwater, C.; Giles, P.; Murray-Lyon, I.M. Khat chewing delays gastric emptying of a semi-solid meal. Aliment. Pharmacol. Ther. 2007, 9, 81–83.
- Tucci, S.A. Phytochemicals in the Control of Human Appetite and Body Weight. Pharmaceuticals 2010, 3, 748–763.
- Chapman, M.H.; Kajihara, M.; Borges, G.; O’Beirne, J.; Patch, D.; Dhillon, A.P.; Crozier, A.; Morgan, M.Y. Severe, Acute Liver Injury and Khat Leaves. N. Engl. J. Med. 2010, 362, 1642–1644.
- Stuyt, R.J.L.; Willems, S.M.; Wagtmans, M.J.; Van Hoek, B. Chewing khat and chronic liver disease. Liver Int. 2011, 31, 434–436.
- Mwenda, J.; M’Arimi, M.M.; Kyama, M.; Langat, D. Effects of khat (Catha edulis) consumption on reproductive functions: A review. East Afr. Med J. 2005, 80, 318–323.
- Dhadphale, M.; Mengech, A.; Chege, S.W. Miraa (Catha edulis) as a cause of psychosis. East Afr. Med. J. 1981, 58, 130–135.
- Critchlow, S.; Seifert, R. Khat-induced Paranoid Psychosis. Br. J. Psychiatry 2018, 150, 247–249.
- Jager, A.D.; Sireling, L. Natural history of Khat psychosis. Aust. N. Z. J. Psychiatry 1994, 28, 331–332.
- Alem, A.; Shibre, T. Khat induced psychosis and its medico-legal implication: A case report. Ethiop. Med. J. 1997, 35, 137–139.
- Ahmed, A.M. Effect of Khat on the Heart and Blood Vessels. Heart Views 2004, 5, 54–57.