Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jingjing Yan and Version 3 by Amina Yu.
Ophiobolins are a group of sesterterpenoids with a 5-8-5 tricyclic skeleton. They exhibit a significant cytotoxicity and present potential medicinal prospects.
- ophiobolins
- unclustered oxidase
- transporter
- compartmentalized biosynthesis
1. Introduction
Terpenoids, with complicated and diverse structures, are the largest category of natural products. Up to date, more than 175,000 kinds of terpenoid-like and terpenoid-derived compounds have been collected in TeroKit (http://terokit.qmclab.com/, accessed on 1 November 2021) [1]. Sesterterpenoids are a class of rare terpenoids containing five isoprenyl units and are mainly isolated from metazoan, plants and fungi [1]. Although the amount of sesterterpenoids only accounts for less than 1.5% of the total terpenoids, many of these compounds show broad-spectrum biological activities and promising application prospects [1]. The 14/18-membered sesterterpenoids synthesized by terpene synthase LcTPS2 from Leucosceptrum canum exhibit significant immunosuppressive activity [2]. Asperterpenols A and B isolated from Aspergillus sp. 085242 strongly inhibit acetylcholinesterase [3][4][3,4]. Nine new sesterterpenoids synthesized by a silent gene cluster in Aspergillus ustus 094102 were identified by gene mining, among which aspergilol A and B showed cytotoxic activities against MCF-7 [5].
Ophiobolins belong to sesterterpenoids with a 5-8-5 tricyclic skeleton. In total, 112 ophiobolins have been discovered so far [6][7][8][9][10][11][12][13][14][15][16][17][18][6,7,8,9,10,11,12,13,14,15,16,17,18], most of which are isolated from fungi of the genus Bipolaris and Aspergillus [6]. Ophiobolins show excellent biological activities, especially cytotoxicity. Ophiobolin A exhibits notable activities against CLL and P388 cell lines [19][20][19,20]. Ophiobolin O can inhibit the proliferation of MCF-7 and reverse the resistance of MCF-7/ADR to adriamycin [21][22][21,22]. The pharmacological mechanism study of 6-epi-ophiobolin G shows that it can be used as a potential estrogen receptor down-regulator to treat breast cancer cells [23].
Though researchers have achieved the total chemical synthesis of (+)-ophiobolin A, (-)-6-epi-ophiobolin N and (+)-6-epi-ophiobolin A, it is severely limited by a high cost and low yield [24][25][26][24,25,26]. The first ophiobolin F (1) synthase AcOS was found from A. clavatus [27]. Following this, an ophiobolin biosynthesis gene cluster (obl) from A. clavatus, Bipolaris maydes producing ophiobolin A and Emericella variecolor (renamed to Aspergillus stellatus) and producing ophiobolin K (2) were reported [28](Figure S1) [28]. In the obl, chimeric terpene synthase OblAAc/Bm/As catalyzes the formation of backbone 1. P450 monooxygenase OblBBm/As is involved in the oxidative modification of 1 to form ophiobolin C (3); then, 6-epi-ophiobolin C (4) and 6-epi-ophiobolin N (5) were formed by non-enzymatic transformation from 3. Moreover, it has been proposed that FAD-dependent oxidoreductase OblCAc catalyzes the C17-allyl formation of 2 in the side chain of 3 [28].
A. ustus 094102 produces a series of ophiobolins (2 and 6–9) (Figure 1) [7][29][7,29]. POC8003 (renamed to oblAu) was verified to be responsible for ophiobolin biosynthesis in A. ustus 094102, which contains au8003 (renamed to oblAAu), au8002 (renamed to oblBAu) and au8001 (renamed to oblRAu) (Figure S1 and Figure 2a) [29]. OblAAu has been identified as ophiobolin F synthase [29], and OblBAu was found as a P450 enzyme that could bind to carbon monoxide [30]. However, no gene was found to exist in oblAu that codes for an enzyme similar to OblC, which may catalyze the formation of a C16=C17 double bond to generate 2. Additionally, the putative transporter OblD that plays an essential role in the synthesis of ophiobolins [28] has not been clarified in oblAu.
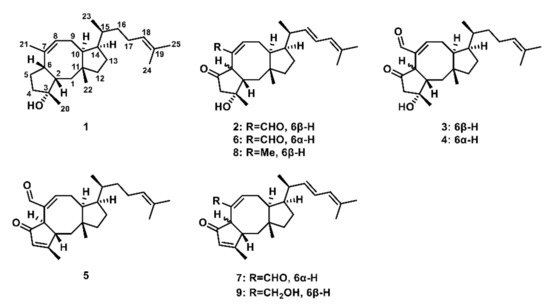
Figure 1. Chemical structure of a part of ophiobolins. Ophiobolin F (1); ophiobolin K (2); ophiobolin C (3); 6-pei-ophiobolin C (4); 6-epi-ophiobolin N (5); 6-epi-ophiobolin K (6); 6-epi-ophiobolin G (7); 21,21-O-dihydro-6-epi-ophiobolin G (8); 21-deoxyophiobolin K (9).
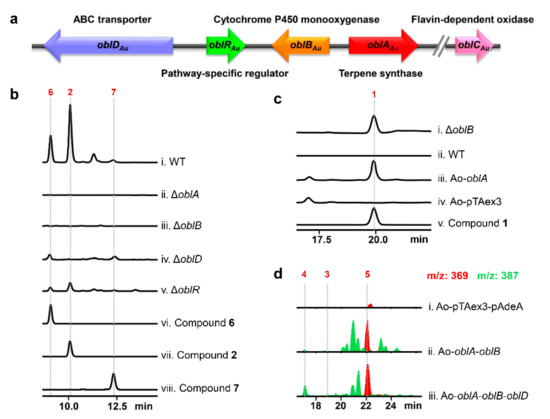
Figure 2. Functional confirmation of genes in ophiobolin biosynthesis gene cluster of Aspergillus ustus 094102 (oblAu). (a) Ophiobolin biosynthetic gene cluster in A. ustus 094102. (b) HPLC profiles of standards and culture extracts from wild-type (WT) strain and mutants at the wavelength of 234 nm. (c) HPLC analysis of WT strain, ΔoblB, Ao-pTAex3 and Ao-oblA at the wavelength of 200 nm. (d) LC–ESI-HRMS analysis of culture extracts from Aspergillus oryzae transformants. Peaks of m/z 369 (red) and m/z 387 (green) were extracted.
2. The Biosynthesis and Transport of Ophiobolins in Aspergillus ustus 094102
Terpenoid biosynthesis gene clusters usually consist of several genes, encoding the terpene synthase for the skeleton [31][34] and oxidoreductases for the hydroxylation, aldehydeylation, carboxylation and dehydrogenation [3][4][32][3,4,35]. In the four reported ophiobolin gene clusters, terpenoid synthase, P450 oxidase and flavin-dependent oxidase jointly completed the synthesis of ophiobolins, while the distribution of these genes was irregular. It wasn our study, we found that the flavin-dependent oxidase OblCAu was unclustered. Similarly, in another ophiobolin K-producing strain A. stellatus, this enzyme was also not in the oblAs. Additionally, even though OblCBm was located in the oblBm of B. maydes, the enzyme responsible for C14-hydroxylation to form ophiobolin A was not found [28]. It is possible that the two enzymes were also unclustered.
It was assumed that OblCAu may have a substrate promiscuity. In A. ustus 094102, OblCAu could dehydrogenate intermediates generated in the multi-step oxidation process (Scheme 1), as it wase discovered 21,21-O-dihydro-6-epi-ophiobolin G (8) and 21-deoxyophiobolin K (9) from its products [7]. Additionally, the docking results of OblC with 1 and 3 further proved this point. In the catalytic pockets of both models, most residues, especially those near the side chains, were the same (Figure S8), which indicated that the side chain was the core site where OblC recognized substrates, and changes of groups at the rings away from the side chain had little effect on the catalytic activity of OblC. Owing to the substrate promiscuity of OblCAu and the non-enzymatic transformation of natural ophiobolins, nearly 20 ophiobolins were found in the metabolites of A. ustus 094102 [7]. FAD-dependent oxidoreductases play an important role in the modification of the terpene skeleton. It has been reported that an unclustered FAD-dependent oxidase EriM responsible for the aldehydation at C-15 in erinacines could catalyze erinacine X, erinacine (14) and erinacine Q to form erinacine ZA, erinacine T and erinacine P, respectively [33][36]. This discovery suggests that there may be more FAD oxidases with substrate promiscuity responsible for terpenoid biosynthesis, which provides critical clues for the discovery of more terpenoids and combinational synthesis of non-natural products.

Scheme 1. Proposed ophiobolin biosynthetic pathway. NET: non-enzymatic transformation.
The compartmentalized biosynthesis of natural products in fungi is a unique mechanism of higher organisms containing organelles. Compartmentalization can restrict different enzymes and substrates to specific subcellular spaces, thereby effectively preventing non-specific reactions and the cytotoxicity of intermediates or products. The biosynthesis of mycophenolic acid derived from Penicillium brevicompactum displays a typical compartmentalization feature, because it relies on the β-oxidation metabolism process in the peroxisome to shorten the isopentenyl long chain specifically [34][37]. In the research, OblDAu transported ophiobolins outside of the cell membrane to realize the self-resistance of hosts against its toxic metabolites.
3. Conclusions
It was confirmed that the flavin-dependent oxidase OblCAu was unclustered and displayed the feature of substrate promiscuity. And it was realized the de novo synthesis of 2 in a heterologous host to build blocks for constructing high-yield ophiobolin engineering strains by using a combinatorial biosynthetic approach.
