You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Béatrice Albert.
Apertures are the areas where the exine is thinner or even lacking. A great diversity in pollen grain morphology is observed in angiosperms.
- cytokinesis
- microsporogenesis
- pollen
- aperture pattern
1. Introduction
Pollen grains, the male gametophytes of flowering plants, are simple organisms composed of two to three cells surrounded by a complex multilayered protective wall made of sporopollenin (outer wall, called exine), and cellulose and pectins (inner wall, called intine). Apertures are the areas where the exine is thinner or even lacking. A great diversity in pollen grain morphology is observed in angiosperms. Variation concerns size, shape, aperture pattern, and pollen wall ornamentation [1]. Aperture pattern is defined as the number, structure, and position of apertures. Apertures may vary in structure (pore, furrow, or both), number (from no aperture to more than one hundred), and position on pollen surface. They are flexible and permeable areas preventing pollen wall breakage during volume variation due to water flows, and they also allow gas exchanges and are thereby strongly involved throughout the processes of fertilization, from pollen survival during pollination to germination of the pollen tube [2].
A wide range of variation in aperture pattern is observed throughout angiosperms [3,4][3][4]. Although variation occurs at any taxonomic level, even down to the intraindividual level [5], large categories can be recognized within angiosperms according to the predominant aperture type. Early-diverging angiosperms and monocots (as gymnosperm) mainly produce monosulcate pollen grains, i.e., a single furrow-shaped aperture located at the distal pole, while eudicots tend to produce tricolpate pollen grains, i.e., three apertures equally distributed along the equatorial region [6].
2. Aperture Formation
Microsporogenesis (male meiosis of seed plants) is the earliest step of pollen development. It consists of nuclear divisions associated with cytoplasmic partitioning (cytokinesis). This process starts from pollen mother cells (or microsporocytes) enclosed in a callose envelope within which meiosis takes place. Cytokinesis is achieved by the formation of callose cleavage walls. Once meiosis is completed, the resulting four microspores form a tetrad embedded within the callose wall of the pollen mother cell, until digestion of the callose by an enzyme called callase. In most species, the apertures are already visible at the late tetrad stage, showing that aperture pattern is determined during microsporogenesis. As mentioned above, the pollen wall is complex and multilayered. The outer wall (exine) is composed of sporopollenin and is structured in several layers each with specific features. The inner wall (intine) is composed of cellulose and pectins. It is apposed to the plasma membrane of the vegetative cell. The apertures correspond generally to areas where the exine is absent or modified and the intine is thicker. Understanding how individual apertures are formed at the microspore stage thus requires understanding how the exine is formed and either inhibited or modified in the areas that will become apertures. Nevertheless, in few cases reported, the aperture develops independently in the intine and in the exine. This the case of inaperturate pollen grains which were found to have no ectoaperture but present endoaperture (Zavata et al., 1997; Pozhidaev, 2003). Several studies have permitted to build a model of exine formation [2,7,8,9,10,11,12,13,14,15,16,17][2][7][8][9][10][11][12][13][14][15][16][17]. The model of exine formation has several steps: (1) at the tetrad stage, the microspores are entirely enclosed in callose, primexine (exine precursor) is deposited between callose and the plasma membrane; (2) the plasma membrane undulates, and structural elements (probaculae) are formed above the protrusions of the undulating membrane; and (3) sporopollenin is then deposited on the microspore surface. It has to be noted that the apertural regions are not mentioned in this model.2.1. Cellular Components Correlated with Aperture Location
At the apertural sites, the absence of exine formation is generally due to an absence of primexine. This absence of primexine could, in turn, be due to the apposition of a plate of endoplasmic reticulum onto the plasma membrane, which prevents the local deposition of primexine, or it could be due to the presence of callosic knobs (also called additional callose deposits) at the places where the apertures are located. The apposition of an endoplasmic reticulum shield against the inner side of the plasma membrane in the apertural region has been observed in a large number of species. These species, which belong to both monocotyledons and eudicotyledons, exhibit various pollen aperture patterns: polyporate pollen grains—Silene pendula [18], monoporate pollen grains—Zea mays [19] and Sorghum bicolor [20], monosulcate pollen grains—Lilium longiflorum [21], tricolporate pollen grains—Tragopogon porrifolius [22], and tricolpate pollen grains—Helleborus foetidus [23] and Helianthus annuus [24].
The presence of callosic knobs or additional callose deposits that could prevent primexine deposition has been observed in several lineages of angiosperms, including early-diverging angiosperms. These additional callose deposits follow cytoplasm partitioning during the formation of microspores. Several callose deposits can occur successively in a species. The localization of the last additional callose deposition is always correlated with the position of the future aperture (Figure 1). Callose deposits related to aperture position were described for the first time in Ipomoea purpurea [25] that produces polyporate pollen grain. Later on, Blackmore and Barnes [23] showed that there is a link between differential tetrad callose wall deposition and primexine localization in Tragopogon porrifolius. Effectively, after the completion of the precisely structured callose wall where the positions of future ridges, spines and apertures are evident, the deposition of primexine begins. Primexine deposition is restricted to developing ridges and spines and is absent from apertural regions. More recently, the existence of a correlation between the location of the last callose deposits and the location of apertures has been demonstrated in an array of species belonging to various families in the major clades of angiosperms (magnoliids, monocots, and eudicots) and with various aperture patterns. This has been described for five eudicot species, namely Grevillea rosmarinifolia [26], Paranomus reflexus (Proteaceae), Epilobium roseum (Onagraceae) [27], Protea lepicarpodendron, and Helleborus foetidus (Ranunculaceae) [28], all of which produce triaperturate pollen grains. This correlation has also been observed in 28 monocot species that produce diporate, monosulcate, trichotomosulcate, tetraporate, and monoporate pollen grains [27,28,29,30,31][27][28][29][30][31]. These species belong to various unrelated orders and families: Butomaceae (Alismatales), Agavaceae, Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae (Asparagales), Liliaceae (Liliales), Bromeliaceae and Typhaceae (Poales), and Pontederiaceae (Pontederiales). Furthermore, among early-diverging angiosperms the species Calycanthus floridus (Calycanthaceae, magnoliids) that produces disulculate pollen grains also exhibits this correlation [30]. Thus, the correlation between the localization of the last additional callose deposition and the position of the future aperture is not linked to a particular aperture pattern.
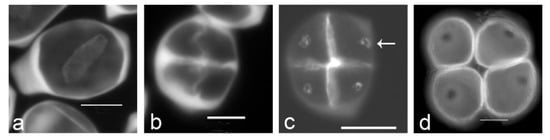

Figure 1. Aperture localization is associated with callose spots in Typha latifolia. (a) Successive cytokinesis with centrifugal cleavage wall formation. (b) Tetragonal tetrad right after cleavage wall formation. (c) Later-stage tetrad with callose spots resulting from additional callose deposition after cleavage wall formation (arrow). (d) Mature tetrad of monoporate pollen grains, callose was dissolved. Tetrads were stained with aniline blue. Scale bars: 10 µm.
Additional alternative ontogenic processes correlated with aperture formation and positioning have been described at the intracellular level in a few species. In Epilobium angustifolium, it has been shown that interstitial bodies are present at the future apertural sites [32]. In Parkinsonia aculeata, the plasma membrane of each microspore presents an irregular pattern except at the apertural site where the membrane is smooth [33]. In Liriodendron tulipifera, Nelumbo nucifera, and Nelumbo lutea, aperture formation is not the result of primexine formation inhibition at the future apertural sites. The microspores of Liriodendron tulipifera are totally enclosed in exine, and the aperture position seems to be determined by an exine fold localized in the distal region of the tetrad. The exine fold breaks down at microspore liberation from the tetrad [34]. In Nelumbo nucifera, Flynn and Rowley [35] state that presumptive apertural areas do not exist in early microspores of this species because primexine is deposited overall the microspore surface, and they could not observe any change that could permit to detect how or when apertures form. The appearance of the colpi might be an example of focal autolysis. In Nelumbo lutea, Kreunen and Osborn [36] describe primexine distribution uniformly around the microspore, and no accumulation of reticulum endoplasmic at the apertural sites. They confirm the post-tetrad establishment of aperture in Nelumbo.
Recent studies have highlighted the role of specific proteins in the process of aperture formation and localization. In Arabidopsis thaliana, rice, and maize, the loss of the INP1 protein causes a complete aperture loss, suggesting a role of INP1 as an aperture factor [37,38,39][37][38][39]. The role of INP1 is conserved in Eschscholzia californica [40]. INP2 has been identified as a partner of INP1 [41], and inp2 mutant displays inaperturate pollen grain. INP1 and INP2 are both proteins of unknown function [41]. The Arabidopsis thaliana protein kinase D6PKL3 is also involved in aperture formation [42]. Indeed, during pollen development, D6PKL3 accumulates at the future aperture sites. The d6pkl3 mutants develop abnormal apertures on the pollen surface, resulting in pollen grains that either lack apertures or, more commonly, have aperture regions that are partially covered with exine [42]. A lectin receptor-like kinase in Oryza sativa, OsDAF1 is also essential for aperture formation [39]. This protein localizes to the future aperture sites at the tetrad stage. Most pollen grains produced by this mutant were aborted, and the surviving pollen grains were inaperturate [39]. A recent study concerning the BcMF8 transgenic line of Brassica campestris [43] has revealed the involvement of the BcMF8 arabinogalactan protein in cell wall development, aperture formation, and pollen tube growth. BcMF8 is a cell-wall-secreted protein which acts to maintain normal intine formation. In the transgenic line, intine is thicker both at the apertural and in non-apertural sites and 80% of pollen grains are tetra-aperturate instead of triaperturate. More information is needed to understand the role of this protein, as microsporogenesis was not described in the paper.
2.2. Determination of Aperture Localization
Cellular mechanisms and the position of cellular components involved in aperture formation in the microspores were explored early in the 20th century by Wodehouse [44] followed by Blackmore and Crane [45] and Ressayre et al. [46]. They suggested that the spatial information determining aperture localization within the tetrad is provided by the last contact points persisting at the end of cytokinesis between the cytoplasms of the future microspores. Ressayre et al. [47] proposed a model that predicts these last contact point positions between microspores. This model is based on the interaction among three meiotic characters: the type of cytokinesis, the tetrad form (which results from the respective orientation of the second meiotic axes), and the way in which callose cleavage walls are formed among the microspores. Cytokinesis type (successive/simultaneous) and tetrad form (tetragonal/rhomboidal/tetrahedral) determines the number and the spatial arrangement of cleavage walls among nuclei. The mode of cleavage wall formation (centrifugal/centripetal callose progression within a cleavage plan), associated with the number and spatial distribution of cleavage walls, and with the timing of cleavage walls formation, determines the areas where the callose is deposited last within the tetrad. These areas are the last contact points among the four microspores. Ressayre et al. [47] furthermore proposed two different ways in which aperture position may be determined. In the first one, the apertures are found at these last contact points among microspores, as suggested by Wodehouse [44] (grouped apertures), while in the second one, the apertures are centered on the distal poles of the microspores and oriented toward these last points (polar apertures) [47]. This model accounts for the localization of apertures only when aperture number is comprised between one and four. The last contact points among microspores are potentially determined by microtubule distribution, which directs the transport of cellular components to the places where apertures should be formed [48].
The developmental model determining aperture localization [47] has been tested by examining the role on aperture pattern of cytokinesis, tetrad form, and callose cleavage wall formation in a large array of species with various aperture patterns.
References
- Heslop-Harrison, J. The Pollen Wall: Structure and Development; Butterworth: London, UK, 1971.
- Edlund, A.F.; Swanson, R.; Preuss, D. Pollen and stigma structure and function: The role of diversity in pollination. Plant Cell 2004, 16, S84–S97.
- Erdtman, G. Pollen Morphology and Plant Taxonomy: Angiosperms (An Introduction to Palynology); Almqvist & Wiksell: Stockholm, Sweden, 1952.
- PalDat. 2021. Available online: http://www.paldat.org/ (accessed on 22 December 2021).
- Mignot, A.; Hoss, C.; Dajoz, I.; Leuret, C.; Henry, J.; Dreuillaux, J.M.; Heberle-Bors, E.; Till-Bottraud, I. Pollen aperture polymorphism in the angiosperms: Importance, possible causes and consequences. Acta Bot. Gall. 1994, 141, 109–122.
- Walker, J.; Doyle, J. The bases of angiosperm phylogeny, palynology. Ann. Mo. Bot. Gard. 1975, 62, 664–723.
- Godwin, H.; Echlin, P.; Chapman, B. The development of the pollen grain wall in Ipomoea purpurea (L.) Roth. Rev. Palaeobot. Palyno 1967, 3, 181–195.
- Vijayaraghavan, M.R.; Shukla, A.K. Absence of callose around the microspore tetrad and poorly developed exine in Pergularia daemia. Ann. Bot. 1977, 41, 923–926.
- PaxsonSowders, D.M.; Owen, H.A.; Makaroff, C.A. A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma 1997, 198, 53–65.
- Doores, A.S.; Osborn, J.M.; El-ghazaly, G. Pollen ontogeny in Ephedra americana (Gnetales). Int. J. Plant Sci. 2007, 168, 985–997.
- Dong, X.; Hong, Z.; Sivaramakrishnan, M.; Mahfouz, M.; Verma, D.P.S. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 2005, 42, 315–328.
- Enns, L.C.; Kanaoka, M.M.; Torii, K.U.; Comai, L.; Okada, K.; Cleland, R.E. Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol. Biol. 2005, 58, 333–349.
- Nishikawa, S.-I.; Zinkl, G.; Swanson, R.; Maruyama, D.; Preuss, D. Callose (b-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol. 2005, 5, 22.
- Suzuki, T.; Masaoka, K.; Nishi, M.; Nakamura, K.; Ishiguro, S. Identification of kaonashi mutants showing abnormal pollen exine structure in Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 1465–1477.
- Zavada, M.S.; Anderson, G.J. The wall and aperture development of pollen from dioecious Solanum appendiculatum: What is inaperturate pollen? Grana 1997, 36, 129–134.
- Pozhidaev, A.E. Hypothetical way of pollen aperture patterning. A family-based study of Krameriaceae. Rev. Palaeobot. Palynol. 2003, 127, 1–23.
- Ariizumi, T.; Toriyama, K. Genetic Regulation of Sporopollenin Synthesis and Pollen Exine Development. Ann. Rev. Plant Biol. 2011, 62, 437–460.
- Heslop-Harrison, J. An ultrastructural study of pollen wall ontogeny in Silene pendula. Grana Palynol. 1963, 4, 7–24.
- Skvarla, J.; Larson, D. Fine structural studies of Zea mays pollen I: Cell membranes and exine ontogeny. Am. J. Bot. 1966, 53, 1112–1125.
- Christesen, J.E.; Horner, H.T. Pollen pore development and its spatial orientation during microsporogenesis in the grass Sorghum bicolor. Am. J. Bot. 1974, 61, 604–623.
- Dickinson, H. Ultrastructural aspects of primexine formation in the microspore tetrad of Lilium longiflorum. Cytobiologie 1970, 1, 437–449.
- Blackmore, S.; Barnes, S. Pollen wall morphogenesis in Tragopogon porrifolius L. (Compositae: Lactuceae) and its taxonomic significance. Rev. Palaeobot. Palynol. 1987, 52, 233–246.
- Echlin, P.; Godwin, H. The ultrastructure and ontogeny of pollen in Helleborus foetidus L. J. Cell Sci. 1968, 3, 175–186.
- Horner, H.T.; Pearson, C.B. Pollen wall and aperture development in Helianthus annuus (compositae: Heliantheae). Am. J. Bot. 1978, 65, 293–309.
- Waterkeyn, L.; Bienfait, A. On a possible function of the callosic special wall in Ipomea purpurea (L.) Roth. Grana 1970, 10, 13–20.
- Albert, B.; Nadot, S. Aperture ontogeny in the Proteaceae Grevillea rosmarinifolia. In Pollen: Structure, Types and Effects; Kaiser, B.J., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010.
- Albert, B.; Matamoro-Vidal, A.; Raquin, C.; Nadot, S. Formation and function of a new pollen aperture pattern in angiosperms: The proximal sulcus of Tillandsia leiboldiana (Bromeliaceae). Am. J. Bot. 2010, 97, 365–368.
- Ressayre, A.; Triky-Teurtroy, S.; Forchioni, A.; Dreyer, L.; Nadot, S. Post-meiotic cytokinesis and pollen aperture pattern ontogeny: Comparison of development in four species differing in aperture pattern. Am. J. Bot. 2005, 92, 576–583.
- Ressayre, A. Equatorial aperture pattern in monocots: Same definition rules as in eudicots? The example of two species of Pontederiaceae. Int. J. Plant Sci. 2001, 162, 1219–1224.
- Albert, B.; Ressayre, A.; Nadot, S. Correlation between pollen aperture pattern and callose deposition in late tetrad stage in three species producing atypical pollen grains. Am. J. Bot. 2011, 98, 189–196.
- Toghranegar, Z.; Nadot, S.; Albert, B. Variation of microsporogenesis in monocots producing monosulcate pollen grains. Ann. Bot. 2013, 112, 135–139.
- Rowley, J.R. Germinal apertural formation in pollen. Taxon 1975, 24, 12–25.
- Larson, D.; Lewis, C. Pollen wall development in Parkinsonia aculeata. Grana 1962, 3, 21–31.
- Guzzo, F.; Baldan, B.; Bracco, F.; Mariani, P. Pollen development in Liriodendron tulipifera: Some unusual features. Can. J. Bot. 1994, 72, 352–358.
- Flynn, J.; Rowley, J.R. The primexine of Nelumbo nucifera. Experientia 1971, 27, 227–228.
- Kreunen, S.S.; Osborn, J.M. Pollen and anther development in Nelumbo (Nelumbonaceae). Am. J. Bot. 1999, 86, 1662–1676.
- Dobritsa, A.; Coerper, D. The novel plant protein inaperturate pollen1 marks distinct cellular domains and controls formation of apertures in the Arabidopsis pollen exine. Plant Cell 2012, 24, 4452–4464.
- Li, P.; Ben-Menni Schuler, S.; Reeder, S.H.; Wang, R.; Suárez Santiago, V.N.; Dobritsa, A.A. INP1 involvement in pollen aperture formation is evolutionarily conserved and may require species-specific partners. J. Exp. Bot. 2018, 69, 983–996.
- Zhang, X.; Zhao, G.; Tan, Q.; Yuan, H.; Betts, N.; Zhu, L.; Zhang, D.; Liang, W. Rice pollen aperture formation is regulated by the interplay between OsINP1 and OsDAF1. Nat. Plant 2020, 6, 394–403.
- Mazuecos-Aguilera, I.; Romero-Garcia, A.; Klodova, B.; Honys, D.; Fernandez-fernandez, M.; Ben-Menni Schuler, S.; Dobritsa, A.A.; Suárez-Santiago, V.N. The role of inaperturate pollen1 as a pollen aperture factor is conserved in the basal eudicot Eschscholzia californica (Papaveraceae). Front. Plant Sci. 2021, 12, 1–14.
- Lee, B.H.; Wang, R.; Moberg, I.; Reeder, S.; Amom, P.; Tan, M.H.; Amstutz, K.; Chandna, P.; Helton, A.; Andrianova, E.P.; et al. A species-specific functional module controls formation of pollen apertures. Nat. Plant 2021, 7, 966–978.
- Lee, B.H.; Weber, Z.T.; Zourelidou, M.; Hofmeister, B.T.; Schmitz, R.J.; Schwechheimer, C.; Dobritsa, A.A. Arabidopsis Protein Kinase D6PKL3 Is Involved in the Formation of Distinct Plasma Membrane Aperture Domains on the Pollen Surface. Plant Cell 2018, 30, 2038–2056.
- Lin, S.; Dong, H.; Zhang, F.; Qiu, L.; Wang, F.; Cao, J.; Huang, L. BcMF8, a putative arabinogalactan protein-encoding gene, contributes to pollen wall development, aperture formation and pollen tube growth in Brassica campestris. Ann. Bot. 2014, 113, 777–788.
- Wodehouse, R. Pollen Grains: Their Structure, Identification and Significance; Medicine, S.a., Ed.; Hafner Publishing Co.: New York, NY, USA, 1935.
- Blackmore, S.; Crane, P. The systematic implications of pollen and spore ontogeny. In Ontogeny and Systematics; Humpries, C.J., Ed.; Columbia University Press: New York, NY, USA, 1988; pp. 83–115.
- Ressayre, A.; Mignot, A.; Siljak-Yakovlev, S.; Raquin, C. Post-meiotic cytokinesis and pollen aperture number determination in eudicots: Effect of the cleavage wall number. Protoplasma 2003, 221, 257–268.
- Ressayre, A.; Godelle, B.; Raquin, C.; Gouyon, P.H. Aperture pattern ontogeny in angiosperms. J. Exp. Biol. (Mol. Dev. Evol.) 2002, 294, 122–135.
- Ressayre, A.; Raquin, C.; Mignot, A.; Godelle, B.; Gouyon, P.H. Correlated variation in microtubule distribution, callose deposition during male post-meiotic cytokinesis, and pollen aperture number across Nicotiana species (Solanaceae). Am. J. Bot. 2002, 89, 393–400.
More
