Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Md Jamal Uddin and Version 3 by Beatrix Zheng.
Both acute and chronic kidney diseases substantially contribute to the morbidities and mortality of patients worldwide. The existing therapeutics, which are mostly developed from synthetic sources, present some unexpected effects in patients, provoking researchers to explore potential novel alternatives. Natural products that have protective effects against various renal pathologies could be potential drug candidates for kidney diseases. Mangiferin is a natural polyphenol predominantly isolated from Mangifera indica and possesses multiple health benefits against various human ailments, including kidney disease.
- chronic kidney disease
- renoprotective
- kidney fibrosis
- oxidative stress
- inflammation
- mangiferin
1. Introduction
Kidney disease is a significant public health problem affecting more than 750 million people worldwide [1]. The burden of kidney disease varies substantially across the world, as does its detection and treatment. Kidney diseases are broadly classified into acute kidney injury (AKI) and chronic kidney disease (CKD). AKI denotes spontaneous kidney damage that generally lasts a few days to a week. The leading causes of AKI are damage to the kidney tissue by drugs or infections, leading to the blockage of urine (for example, a blockage can also be caused by kidney stones) [2]. If these conditions persist, kidney function decreases over time, leading to CKD. In the worst-case scenario, end-stage renal disease (ESRD), also known as kidney failure, can develop. There are many other factors or conditions involved in CKD, such as diabetes mellitus, glomerulonephritis, nephrosclerosis, and polycystic kidney disease [2]. There are many different causes of kidney disease, and sometimes the cause is unknown.
Renoprotective effects imply preserving the kidney structure or function in different pathological conditions. To date, there is no specific treatment option to completely inhibit the progression of AKI and CKD by preserving the integrity of architecture or function of kidneys. However, therapies in terms of kidney protection to confront the risk factors are worthy of consideration for maintaining the patient’s kidney function. A plethora of traditional treatments using natural products have shown a wide range of therapeutic windows for kidney diseases. Among these, one of the exemplary candidates is mangiferin (molecular formula: C19H18O11; systematic name: 1,3,6,7-tetrahydroxyxanthone C2-β-D-glucoside) (Figure 1), a naturally occurring glucoxilxanthone [3][4][3,4] derived from the various parts of Mangifera indica (Mango), including the leaves, fruits, flowers, seeds, roots, and stem bark [5]. In China, mangiferin is included in many traditional formulae that contain Iris domestica, Folium pyrrosiae, Gentiana scabra, and A nemarrhena asphodeloides. Moreover, this natural bioactive and polyhydroxy polyphenol element is enriched with several pharmacological beneficial effects without any known side effects [6].
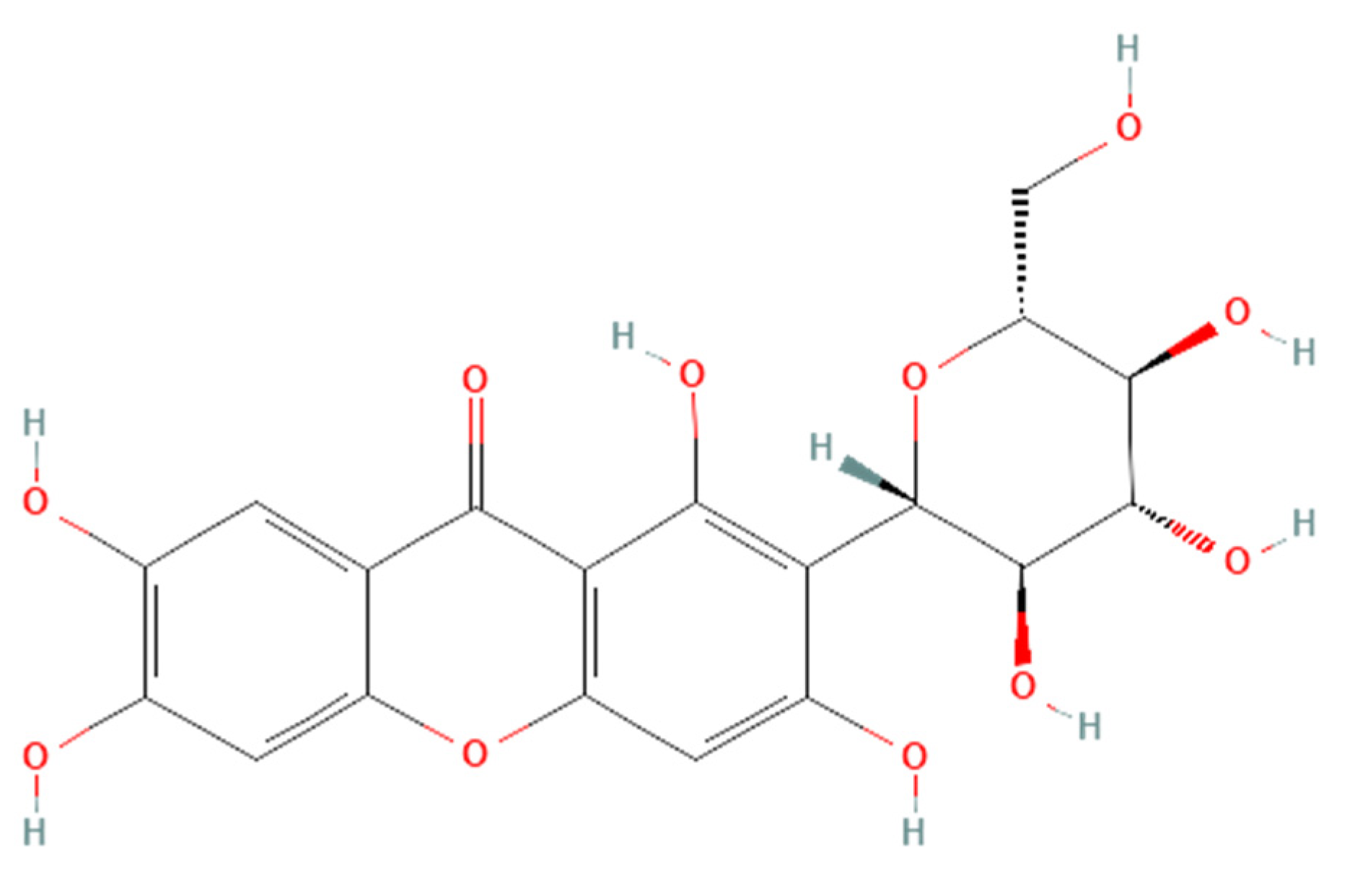
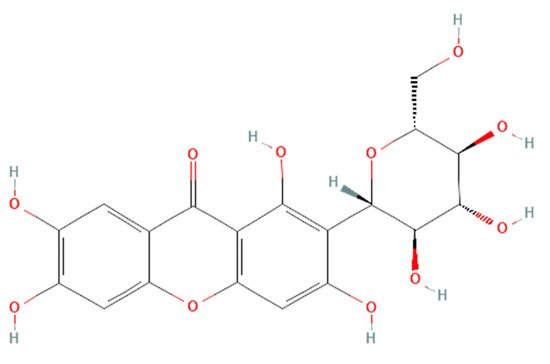
Figure 1. Structure of mangiferin (PubChem CID 5281647).
Mangiferin is shown to have a renoprotective effect. Therefore, it has been extensively investigated regarding the beneficial effects of kidney diseases. Several reports support that mangiferin confers its renoprotective effects against AKI and CKD predominantly through protection against inflammation, scavenging of reactive oxygen species (ROS) in oxidative stress, anti-apoptotic and anti-fibrotic effects in renal intestinal fibrosis, preserving mitochondrial function, and reducing lipid peroxidation [7][8].
Structure of mangiferin (PubChem CID 5281647).
Mangiferin is shown to have a renoprotective effect. Therefore, it has been extensively investigated regarding the beneficial effects of kidney diseases. Several reports support that mangiferin confers its renoprotective effects against AKI and CKD predominantly through protection against inflammation, scavenging of reactive oxygen species (ROS) in oxidative stress, anti-apoptotic and anti-fibrotic effects in renal intestinal fibrosis, preserving mitochondrial function, and reducing lipid peroxidation [7,8].
2. Pharmacological Effects of Mangiferin on Kidney Diseases
The pharmacological potential of mangiferin against several plausible factors such as oxidative stress, inflammation, fibrosis, and other pathologies associated with kidney diseases are summarized in this section (
Figure 2
,
Figure 3
and
Figure 4
and
Table 1
and
Table 2).

).
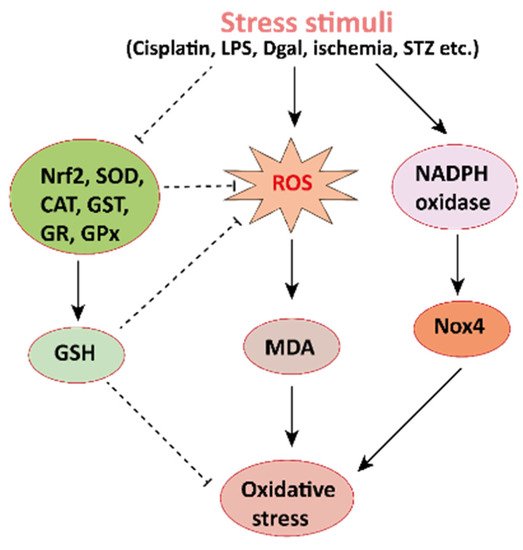

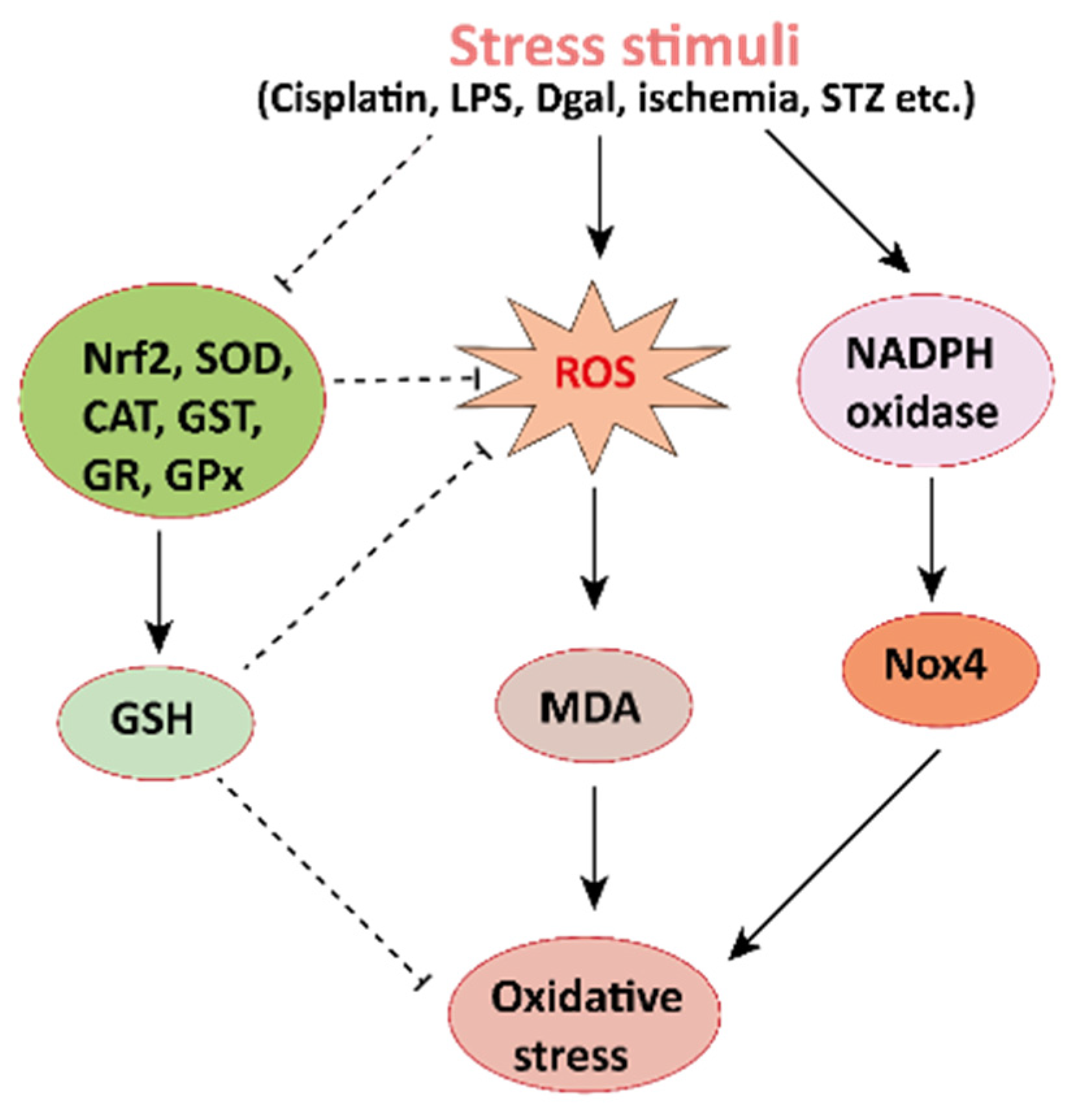
Figure 2. Mechanisms involved in the pathogenesis of oxidative stress in the kidney. NADPH oxidase is the main source of cellular ROS. Nox4 is an isoform of NADPH oxidase expressed in renal tubules that leads to oxidative stress (ROS and MDA) and damages the kidney. Stress stimuli, for instances cisplatin, STZ, ischemia result in decreased Nrf2 thus leading to oxidative stress. Reactive oxygen species, ROS; MDA, malondialdehyde; streptozotocin, STZ; NF-E2-related factor 2, Nrf2.

Mechanisms involved in the pathogenesis of oxidative stress in the kidney. NADPH oxidase is the main source of cellular ROS. Nox4 is an isoform of NADPH oxidase expressed in renal tubules that leads to oxidative stress (ROS and MDA) and damages the kidney. Stress stimuli, for instances cisplatin, STZ, ischemia result in decreased Nrf2 thus leading to oxidative stress. Reactive oxygen species, ROS; MDA, malondialdehyde; streptozotocin, STZ; NF-E2-related factor 2, Nrf2.
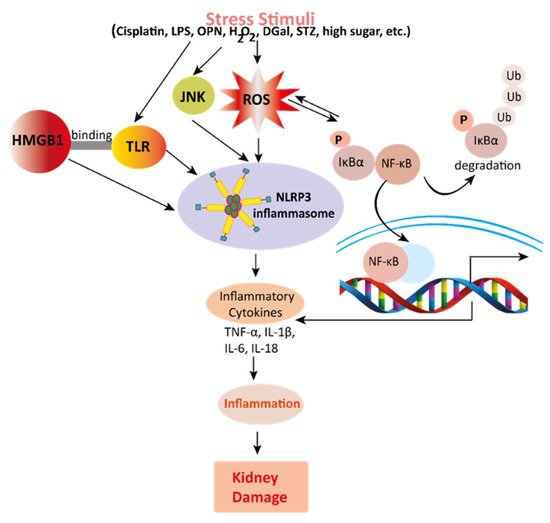
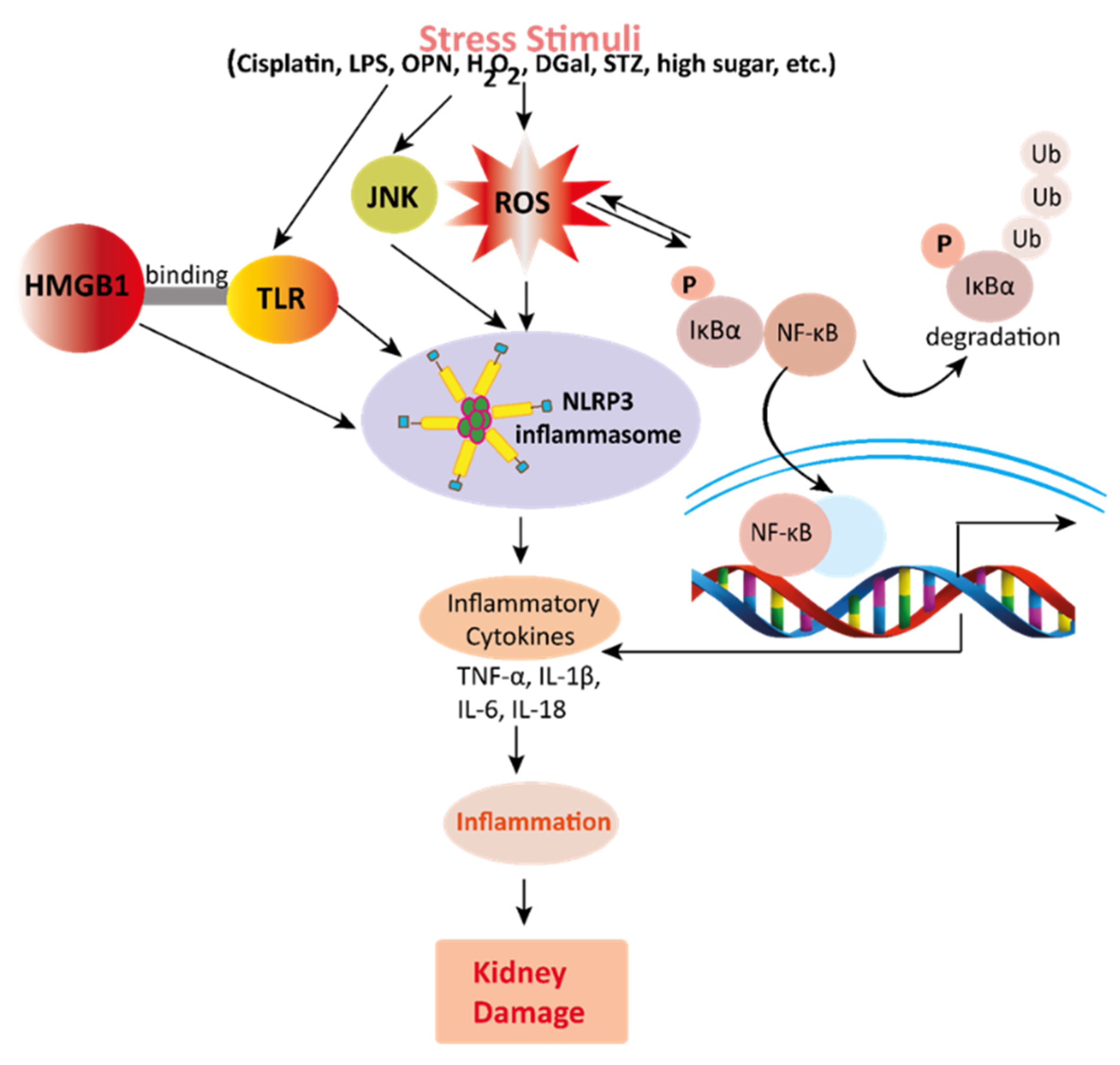
Figure 3. Mechanisms involved in the pathogenesis of inflammation in the kidney. Stress stimuli example for, cisplatin, DGal, STZ, LPS, OPN, high glucose, and H2O2 generate ROS in the kidney. Accumulation of ROS induces inflammation through the activation of the NLRP3 inflammasome. NLRP3 involves a multi-protein complex known as inflammasome, which triggers the NF-κB signaling pathway. HMGB1 protein is a late inflammation instigating compound which activates the NF-κB signaling pathway by binding with Toll-like receptors. The NF-κB pathway further promotes an inflammatory storm by releasing inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-18), which ultimately leads to kidney damage. High-mobility group box 1; HMGB1, D (+) galactosamine; DGal, osteopontin; OPN, Streptozotocin; STZ, reactive oxygen species; ROS, IL-6; interleukin-6, IL-1β; Interleukin 1β, TNFα; Tumor Necrosis Factor-α.

Mechanisms involved in the pathogenesis of inflammation in the kidney. Stress stimuli example for, cisplatin, DGal, STZ, LPS, OPN, high glucose, and H2O2 generate ROS in the kidney. Accumulation of ROS induces inflammation through the activation of the NLRP3 inflammasome. NLRP3 involves a multi-protein complex known as inflammasome, which triggers the NF-κB signaling pathway. HMGB1 protein is a late inflammation instigating compound which activates the NF-κB signaling pathway by binding with Toll-like receptors. The NF-κB pathway further promotes an inflammatory storm by releasing inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-18), which ultimately leads to kidney damage. High-mobility group box 1; HMGB1, D (+) galactosamine; DGal, osteopontin; OPN, Streptozotocin; STZ, reactive oxygen species; ROS, IL-6; interleukin-6, IL-1β; Interleukin 1β, TNFα; Tumor Necrosis Factor-α.
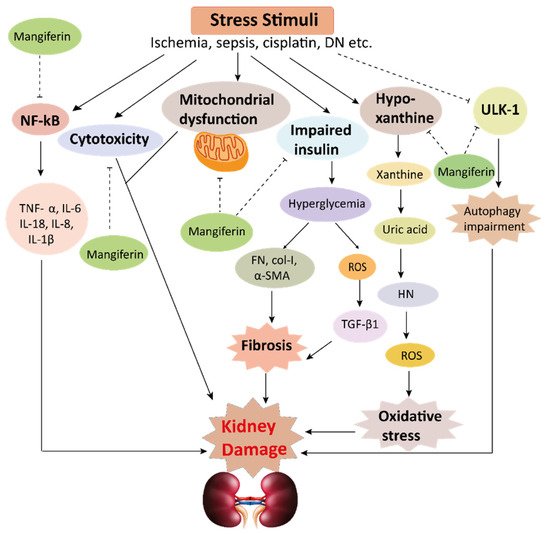
Figure 4.
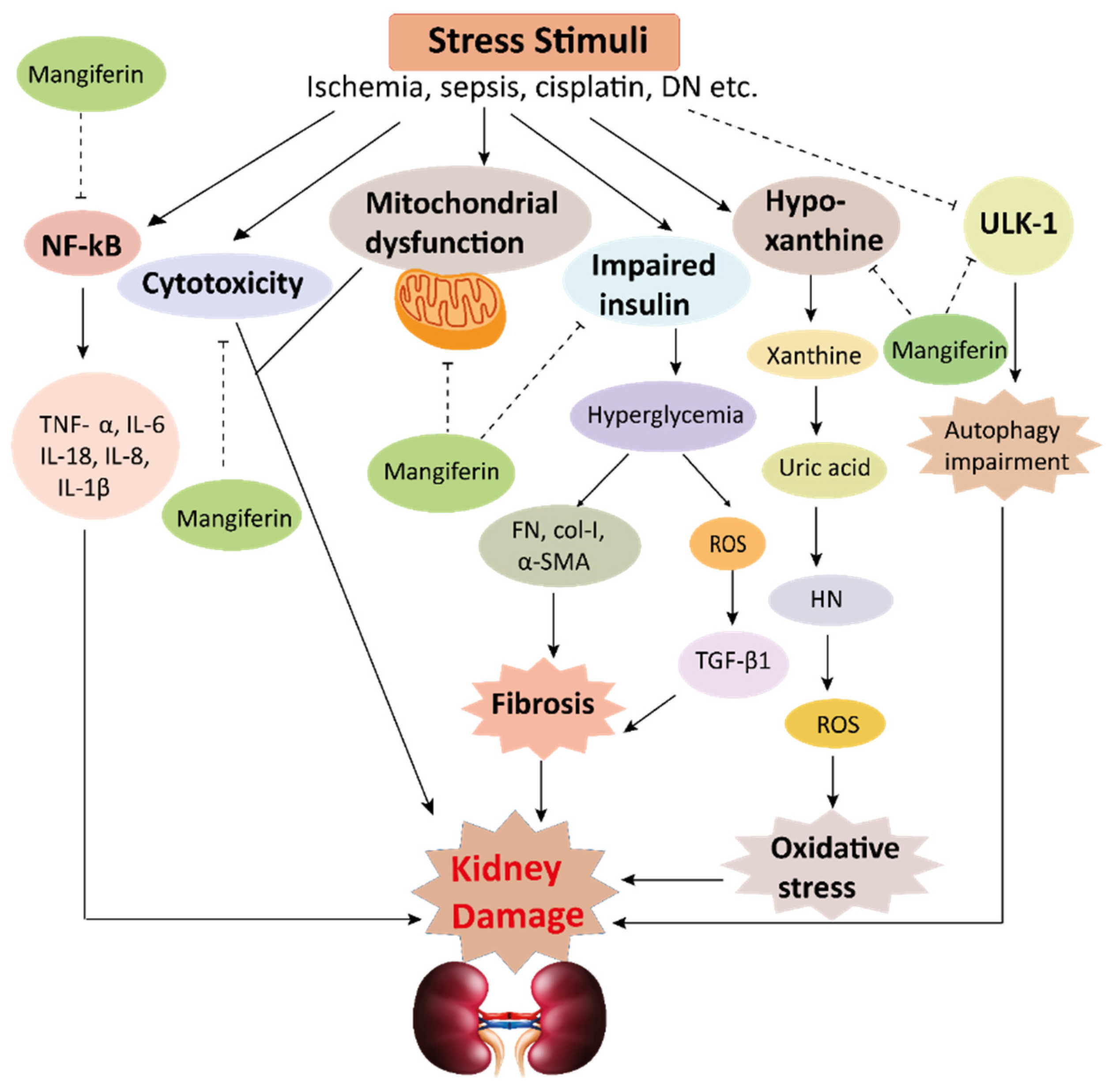
This schematic representation shows that stress stimuli example for, cisplatin, DN, Ischemia, and sepsis mediates various pathological conditions including cytotoxicity, oxidative stress, inflammation, fibrosis, autophagy dysfunction, and mitochondrial dysfunction. These ultimately lead to kidney damage. Stress stimuli activate the NF-kB signaling pathway which triggers the release of inflammatory cytokines (TNF-α, IL-6, IL-18, IL-8, and IL-1β) and decreases the action of ULK-1 thus autophagy impairment causes. Further col-1, FN, α-SMA causes accumulation of extracellular matrix (ECM) resulting in fibrosis. Mangiferin protects the kidney by suppressing the cascades of inflammatory pathways, oxidative stress, fibrosis, cytotoxicity, mitochondrial dysfunction, and autophagy impairment. ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; FN, fibronectin; α-SMA, α-smooth muscle actin; Col I, collagen I; TGF-β1, transforming growth factor, ULK-51, unc-51-like kinase; DN, diabetic nephropathy; HN, hyperuricemic nephropathy; NF-kB, nuclear factor-kappa B.
Table 1. In vivo renoprotective effects of mangiferin.
| Model Animals | Disease Inducing Agents | Mangiferin Dosages | Effects of Mangiferin | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Dose | Route | Duration | Oxidative Stress | Inflammation | Fibrosis | Other Pathologies | |||
| Mice | Cisplatin | 10, 20 and 40 mg/kg | |||||||
| i.p. | |||||||||
| 30 min | |||||||||
| − | |||||||||
| ↓ TNF-α and IL-1β | |||||||||
| − | |||||||||
| ↓ Caspase-3 | |||||||||
| [ | |||||||||
| 25 | |||||||||
| ] | |||||||||
ROS, reactive oxygen species; SOD, superoxide dismutase; GSH, glutathione; CAT, catalase; GST, glutathione S-transferase; GR, glutathione reductase; GPx, glutathione peroxidase; AGEs, advanced glycation end products; α-SMA, α-smooth muscle actin; DGal, D+galactosamine; ERK, extracellular signal-regulated kinases; FN, fibronectin; GLUT9, glucose transporter 9; HMGB1, high-mobility group box 1; i.p, intraperitoneal injection; IL-1β, interleukin 1β; IL-6, interleukin-6; JNK, c-Jun N-terminal kinases; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinases; MDA, malondialdehyde; mTOR, mammalian target of rapamycin; OAT10, organic anion transporter 10; OPN, osteopontin; PI3K, phosphoinositide 3-kinase, TNFα; tumor necrosis factor-α; TGF-β1, transforming growth factor-β1, tBHP; tert-Butylhydroperoxide, ULK1; unc-51-like kinase 1, URAT1; urate-anion transporter 1, VEGF; Vascular endothelial growth factor, XOD; Xanthone Oxidase.
In vivo renoprotective effects of mangiferin.
Table 2. In vitro renoprotective effects of mangiferin.
| Cell Lines | Model Drug | Mangiferin | Effects of Mangiferin | Ref. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Duration | Oxidative Stress | Inflammation | Other Pathologies | |||||||||||
| NKE cells | Cisplatin | 5–30 µMorally | 21 days | ↓ ROS ↑ GSH, SOD, CAT, GST, GR, GPx |
↓ TNF-α, IL-1β, IL-6, IL-10, NF-κB |
2 h | ↓ ROS ↑ GSH, SOD, CAT, GST, GR,− |
GPx |
− | ↓ Caspase-3 | [↓ Caspase-3 | 9][9] | |||
| Rats | Cisplatin | 20 and 40 mg/kg | i.p. | 10 days | ↓ ROS, MDA ↑ GSH, SOD, CAT |
↓ TNF-α, IL-6 | |||||||||
| NKE Cells | − | ↓Bax, caspase-3 | ↑ Bcl-2↓ MAPK pathway | [ | NaF10] | ||||||||||
| 100–1000 μg/mL | ↓ ROS ↑ CAT, peroxidase and GHS |
− | − | [26] | Mice | STZ | 15, 30 and 60 mg/kg/day | orally | 4 Weeks | ↓ ROS, MDA ↑ SOD, CAT, andGSH-Px |
↓TNF-α, IL-6, IL-1β | ↓TGF-β1, FN, Col I, and α-SMA |
↓Phosphorylation of PI3K and Akt | [7] | |
| Rats | STZ | 10, 20, 40, 60 and 80 mg/kg; | orally | 30 days | ↓ ROS, MDA ↑ SOD, CAT, GPX, GR |
↓ NF-kB, TNF-α | − | ↓ Cytochrome C ↓ Bax, Caspase-9, Caspase-3↑ Bcl-2↓ MAPK pathway |
[11] | ||||||
| Rats | STZ | 40 mg/kg | orally | 28 days | ↓ ROS | ||||||||||
| HRGEC | Cadmium | 75 μM | 24 h | ↓ ROS, MDA ↑ SOD, GSH, GR, GSH-Px |
↓ NF-kB, IL-6, IL-8 | ↓ Bax, Cytochrome C, Caspase ↑ Bcl-2 |
[27 ↑ CAT, GST, GS, GSH, GPx, SOD |
↓ NF-κB, TNF-α, VEGF, PKC |
↓ TGFβ1 | − | [12] | ||||
| ] | |||||||||||||||
| Mesangial Cells (SV40 MES 13) | High glucose (25 mM) | 50 mg/kg | 48 hr | Rats | STZ | 12.5, 25, or 50 mg/kg | orally | 12 weeks | − | − | − | ↑ AMPK ↓ mTOR ↑ pULK1 |
[13] | ||
| ↓ ROS | ↓ NOX4 | − | ↓ Caspase-3 ↑ Mitochondrial membranepotential |
[28 | Rats | STZ | 40 mg/kg/day | orally | 30 days | ↑ SOD, CAT, GPx and GSH ↓ ROS, MDA |
− | − | − | [14] | |
| ] | Rats | STZ | 10 and 20 mg/kg | i.p | 28 days | ↓ ROS, MDA ↑ SOD, CAT |
− | − | − | [15] | |||||
| Rats | STZ | 15, 30, and 60 mg/kg | orally | 9 weeks | ↓ ROS, MDA, AGEs ↑ GSH |
− | − | − | [16] | ||||||
| Mice | LPS | 20, 50, and 100 mg/kg | i.p. | 7 days | ↓ ROS, MDA ↑ SOD |
↓ NF-κB, HMGB1 | − | − | [17] | ||||||
| Rats | LPS | 10, 50, 100 μM | 1 h | ↓ ROS, MDA | ↓ IL-1β, IL-18 and NLRP3 |
− | ↓ caspase-9 and caspase-3 |
[18] | |||||||
| Mice | Uric acid | 50 mg/kg/day | orally | 7 days | ↓ ROS, XO ↑ SOD |
↓ IL-1b, IL-18 | − | − | [19] | ||||||
| Mice | Uric Acid | 10, and 30 mg/kg | 7 days | ↓ ROS, MDA | − | − | − | [20] | |||||||
| Rats and mice | Uric acid | 1.5–6.0 mg/kg | i.p. | 5 days | − | − | − | ↓ URAT1, OAT10, and GLUT9 | [21] | ||||||
| Rats | OPN | 15, 30, and 60 mg/kg/day | orally | 9 weeks | − | ↓ TNF-α, COX-2, IL-1β, NF-κB, p65 |
↓ Col IV, α-SMA |
− | [22] | ||||||
| Mice | tBHP | 75 mg/kg | orally | 2 weeks | ↑ SOD, CAT, GST, GRGPX | ↓ TNF-α, IL-6 and IL-1β |
− | ↓ Bax, caspase-8 caspase-3 ↑ Bcl-2 |
[23] | ||||||
| Rats | DGal | 25 mg/kg | i.p. | 14 days | ↓ ROS, MDA ↑ CAT, GST, GS, GPx, SOD |
↓ NF-κB a, NO, TNF-α |
− | ↓ Bax, cytochrome c, caspase-9 and caspase-3 ↑ Bcl-2, Bcl-xL |
[24] | ||||||
| Mice | Ischemia | 10, 30, and 100 mg/kg | |||||||||||||
2.1. Effects of Mangiferin on Renal Oxidative Stress
Oxidative stress arises in the cells owing to an imbalance between production and accumulation of ROS, and subsequent inability to detoxify these reactive products ( In vitro renoprotective effects of mangiferin.
2.1. Effects of Mangiferin on Renal Oxidative Stress
Oxidative stress arises in the cells owing to an imbalance between production and accumulation of ROS, and subsequent inability to detoxify these reactive products (
Figure 2) [29][30][31]. ROS is generated in the cells during the normal process of metabolism. Although optimum ROS level helps cell metabolism by cell-to-cell interactions, elevated ROS can cause extreme damage to cells and tissues [32]. The antioxidative defense mechanism of cells combined with an exogenous source of antioxidants is the key to minimizing the damages occurred by oxidative stress. Mangiferin, the natural bioactive compound has already been proven to have antioxidant properties. It may maintain a proper balance between ROS and antioxidants by reducing the level of intracellular ROS. It increases antioxidant activities in the kidney tissues, and subsequently stabilizes superoxide dismutase (SOD) activities, decreases uric acid synthesis, and improves antioxidant effects in mice and rats (
) [40,41,42]. ROS is generated in the cells during the normal process of metabolism. Although optimum ROS level helps cell metabolism by cell-to-cell interactions, elevated ROS can cause extreme damage to cells and tissues [43]. The antioxidative defense mechanism of cells combined with an exogenous source of antioxidants is the key to minimizing the damages occurred by oxidative stress. Mangiferin, the natural bioactive compound has already been proven to have antioxidant properties. It may maintain a proper balance between ROS and antioxidants by reducing the level of intracellular ROS. It increases antioxidant activities in the kidney tissues, and subsequently stabilizes superoxide dismutase (SOD) activities, decreases uric acid synthesis, and improves antioxidant effects in mice and rats (
Table 1
).
Mangiferin reduces the damage of antioxidants in lipopolysaccharide (LPS)-induced sepsis, thus, recovering sepsis-associated organ impairment [17] and increases antioxidant biomarkers in mouse kidney cells [18]. Besides, it has also been reported that mangiferin reduces intracellular MDA levels by acting as an effective ROS scavenger. Consistently, mangiferin ameliorated the oxidative stress in animal models with different ROS inducers namely uric acid, osteopontin, tert-Butylhydroperoxide (tBHP) and D(+)galactosamine (DGal) [19][20][21][22][23][24] as well as in different kidney cells [26][27][28], as briefly described in
Mangiferin reduces the damage of antioxidants in lipopolysaccharide (LPS)-induced sepsis, thus, recovering sepsis-associated organ impairment [28] and increases antioxidant biomarkers in mouse kidney cells [29]. Besides, it has also been reported that mangiferin reduces intracellular MDA levels by acting as an effective ROS scavenger. Consistently, mangiferin ameliorated the oxidative stress in animal models with different ROS inducers namely uric acid, osteopontin, tert-Butylhydroperoxide (tBHP) and D(+)galactosamine (DGal) [30,31,32,33,34,35] as well as in different kidney cells [37,38,39], as briefly described in
Table 1
and
Table 2. Collectively, all these data from in vivo and in vitro studies indicated that mangiferin could be a potent therapeutic agent to combat oxidative stress in kidney diseases.
2.2. Effects of Mangiferin on Renal Inflammation
. Collectively, all these data from in vivo and in vitro studies indicated that mangiferin could be a potent therapeutic agent to combat oxidative stress in kidney diseases.
2.2. Effects of Mangiferin on Renal Inflammation
2.3. Effects of Mangiferin on Renal Fibrosis
R
2.3. Effects of Mangiferin on Renal Fibrosis
Persistenalt inflammaccumulation is initiated by several factors including immune-mediated inflammatory mediators and a subsequentof extracellular matrix (ECM) in the renal dysfunction or nephrotoxicity (Figure 3) [34][35][36][37][38]. Moreover, intissue creased uric acid triggers renal inflammation through the c-Jun N-terminal kinases (JNK) signaling pathway and nucleotide-binding oligomerization domain (NOD)-, leucine-rich repeat (LRR)- and pyrin domain-containing protein (NLRP)uses kidney fibrosis. ROS helps to intensify the accumulation of ECM by inducing transforming growth factor (TGF)-β1, which is evident in the in vivo mice models [7,50,51,52]. 3 infCellulammasome. NLRP3 inflammasome stimulates pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18 productr ROS also leads to activate fibrotic factors such as fibronectin (FN), α-smooth muscle actin (α-SMA), and collagen I (Col I) (Figure 4). FN ions and excretion, thus promoting renal injuries and disease in mice such as hyperuricemic nephropathy (HN) [19]. I non-collagenous glycoprotein present in ECM and basement membran connection with these, recent studies demonstrated the anti-inflammatory effects of mangiferin in kidney tissues and cells (Table 1 , which helps cell adhesion and regulates cell polarity, differentiation, and enlargemendt, Table 2). Mechanistically,d mangiferin shows its anti-inflammatory effects by inhibitingprevents to activate FN [7]. tThe activation of both the JNKe protein kinase C beta (PKCβ) pathway and NLRP3 inflammasome and minimizing uratecontributes [18]. It has also demonstrated that mangiferin treatment preserved the glomerular and tubular structures of kidneys in renal fibrosis in hyperuricemia boosted mice [19][30]. PKCisplatin manifests nephrotoxicity in the liver is a family of enzyme threonine kinases along with deleterious effects in the renal tissues. Cisplatin exposure escalates proinflammatory cytokines (tumor necrosis factor-α; TNF-α, IL-1β, IL-6, IL-10) and nuclear12 isoforms. Activation of PKC isoforms enhances the vasoendothelial growth factor-kappa β (NF-κB) in the nuclear fraction of the renal tissue in rats and mice; however, mangiferin treatment attenuated the level of all these cytokines [9][10].
Studies (VEGF), leading to Col IV deposition and mesangial growth in demonstrated thats [23]. highOPN mobility group box 1 (HMGB1), a is known as ECM protein that binds to toll-like receptors (TLR), activates NF-κB inflammatory signaling pathways inducing the release of inflammatory factors; hence, it contributes to an inflammatory storm in LPS-treated mice,, and pro-fibrotic adhesion compound induces renal disease tubulointerstitial fibrosis. OPN can act as a mediator for fibrotic changes related to glomerulosclerosis and interestinglystitial fibrosis, however, mangiferin preventreatment attenuated the activationdevelopment of this signaling pathway [17]. Mese pathorelover, simultaneous administration of mgical changes in kidneys [33].
Mangiferin redumay aced cisplatin-instigated nephrotoxicity in vitro and in vivo by preventing the nuclear translocation of NF-κB and pt as a new therapeutic agent against chronic fibrotic kidney diseases such as HN and DN [32]. Troinfleammatory cytokines. Blocking NF-κB pathway by tment with mangiferin defeatimpedes the NF-κB cascade pathway of inflammation in rats and mice [9][10]. Iexpression contrast, tBHP instigated inflammation [23], as wf FN in hypell as STZ rurinduced-diabeticemic nephropathy (DN) in mice [7][11][12][13][14][15][16][30]. wEvidere ameliorated by nce shows that mangiferin. Moreover, DGal advances inflamm administration by prompting NF-κB and TNFα in rats ineffectively shows anti-fibrotic effects against renal tissue, and osteopontin (OPN), a proinflammatory cytokine, assists in inflammatory gene expression. Mangiferin preserves renal function appositely against NLRP3 inflammasomeinterstitial fibrosis by decreasing TGF-β1-induced elevation of Col I, FN, and α-SMA and by inhibiting glomerular [22]ECM and lessens inflammation by obstructing DGal [24] evelopment, accumulation, reducing glomerulandr OPN [22]. In dibabsemetic nephropathy, high glucose generates an inflammatory response characterized by activating the NF-κB pathway, TNF-α, and IL-1β in the renal tissue of nt membrane thickness, as well as mesangial cell growth in STZ, instigated diabetic rats, and mangiferin wasmice [7]. Table to reduce the burden of inflammation in DN [28]. Moreoverken together, mangiferin inhicould bits renal ischemia-reperfusion damages by blocking inflammatory agents [25] ecome a potentiandl preserves renal function against cadmium-induced eukaryotic cell death via the NF-κB signaling pathways [27]. All data suggtherapeutic option to inhibit thest that mangiferin has the ability to combat inflammation in thefibrotic changes in kidneys.
