The hard diagnosis and treatment of NSCLC, together with its late diagnosis and lack of therapies during the early stages of the disease, so far explain its high mortality rate. The reformulation of conventional therapies as nanomedicines, has led to a new generation of treatments for NSCLC. However, the deepening of the relationship between the tumor and the patient's immune system encompasses the most promising strategies today. From immune checkpoint inhibitors, identified in 2018, to the development of vaccines based on genetic material, immunotherapies represent the best of options. The use of nanometric vehicles for the release of genetic material, protein or drug allows reducing doses and increasing efficiency. These, together with delivery systems based on nanomedicines, promise to increase the specificity and efficacy of treatments, reducing the extremely harmful side effects of conventional therapies.
Non-small cell lung cancer (NSCLC) remains the most common cause of cancer-related mortality. The heterogeneous nature of this disease hinders its diagnosis and treatment, requiring continuous advances in research aiming to understand its intricate nature. Consequently, the retrospective analysis of conventional therapies has allowed the introduction of novel tools provided by nanotechnology, leading to considerable improvements in clinical outcomes. Furthermore, the development of novel immunotherapies based on the recently understood interaction of the immune system with the tumor highlights the real possibility of definitively treating NSCLC from its early stages. Novel engineering approaches in nanomedicine will enable to overcome the intrinsic limits of conventional and emerging therapies regarding off-site cytotoxicity, specificity, resistance mechanisms, and administration issues. The convergence point of these therapies with nanotechnology lays the foundation for achieving currently unmet needs.
- drug delivery
- gene delivery
- immunotherapy
- nanovaccines
1. Introduction
Non-small cell lung cancer (NSCLC) remains the most common cause of cancer-related mortality. The heterogeneous nature of this disease hinders its diagnosis and treatment, requiring continuous advances in research aiming to understand its intricate nature. Consequently, the retrospective analysis of conventional therapies has allowed the introduction of novel tools provided by nanotechnology, leading to considerable improvements in clinical outcomes. Furthermore, the development of novel immunotherapies based on the recently understood interaction of the immune system with the tumor highlights the real possibility of definitively treating NSCLC from its early stages. Novel engineering approaches in nanomedicine will enable to overcome the intrinsic limits of conventional and emerging therapies regarding off-site cytotoxicity, specificity, resistance mechanisms, and administration issues. The convergence point of these therapies with nanotechnology lays the foundation for achieving currently unmet needs.
- Introduction
Lung cancer is the major cause of cancer death and one of the leading causes of death worldwide. In 2018, it accounted for more than 2 million deaths, according to the data reported by the World Health Organization (WHO) [1]. Furthermore, statistical studies predict the doubling of its prevalence in the coming years. The American Cancer Society estimates more than 130,000 deaths only in the United States during 2020 [1][2][1,2]. A prospective evaluation of the incidence trend suggests that smoking habits are closely related to the appearance of lung cancer, although other environmental and genetic factors are also determinant. A remarkable increase of lung cancer cases in women has been noticed due to the rise in the number of smokers as a result of social changes, whereas the number of male smokers has traditionally been high [3][4][3,4]. Furthermore, patients suffering from this type of cancer are considered to be at high risk of worse outcomes when suffering from other respiratory conditions, such as chronic obstructive pulmonary disease (COPD) and severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), leading to a higher number of indirect deaths [5][6][7][8][5–8].
Early diagnosis is a must for providing appropriate prognosis and treatment options, thus explaining the importance of correct lung cancer evaluation. The first classification of lung cancer was provided at the beginning of the last century [9] and has been continuously modified to respond to the increase in the number of cases as well as to the identification of new subgroups of cell lung cancer [10]. Currently, the WHO differentiates several types of lung cancer which can be mainly classified into Small Cell Lung Cancer (SCLC) and Non-Small Cell Lung Cancer (NSCLC) (Figure 1). SCLC is diagnosed in the older population, current, former, or second-hand smokers and represents approximately 20% of all cases of lung cancer [11]. NSCLC affects a much broader range of the population and accounts for 80% of all lung cancer cases. This type of cancer includes adenocarcinoma, squamous cell carcinoma, and different histotypes of large cell carcinoma (Table 1) [12][13][14][12–14]. Besides tumor classification, the procedure to be followed is decided based on the accurate staging of lung cancer, which largely depends on the histological and genetic characteristics of the patient [15][10,13,15]. The actual staging system is based on Tumor–Node–Metastasis (TNM) evaluation, regarding the size of the primary tumor, the spreading of cancer cells to the lymph nodes, and the tumor metastatic capacity [16][10,16] (Table 2). Considering metastasis is crucial for NSCLC patients, since approximately 30–40% of patients show tumor migration to other organs, most commonly the bones or the brain [17][18][17,18].
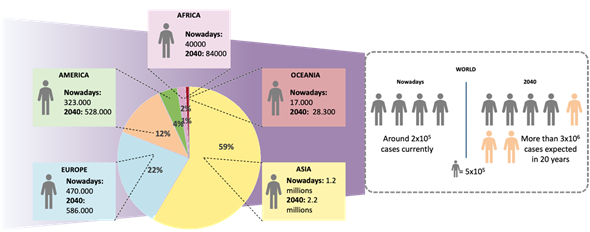
Figure 1. Lung cancer statistics nowadays. Geographical distribution of lung cancer cases and estimated number of cases worldwide in 2018 and 2040 (Source: Globocan).
Table 1. Subclassification of histological heterogeneity of Non-Small Cell Lung Cancer (NSCLC).
|
NSCLC Subtype |
Percentage (% Over NSCLC Cases) |
Characteristics |
Ref. |
|
Adenocarcinoma |
40 |
Presents frequent histologic heterogeneity. Mainly affects the outer edges of the lung. |
[19] |
|
Squamous cell carcinoma |
30 |
Centrally located in the larger bronchi of the lung. The incidence is linked with smoking more than for other NSCLC cancers. |
[19] |
|
Large cell carcinoma |
10 |
Non-differentiated type of lung cancer that lacks the architecture of squamous or glandular differentiation. It usually affects the peripherical part of the lung. |
[19] |
|
Adenosquamous carcinoma |
<5 |
It is a subtype presenting components of both adenocarcinoma and squamous cell carcinoma. |
|
|
Sarcomatoid carcinoma |
<1 |
Centrally located in the larger bronchi of the lung and the peripherical part of the lung. Hard to diagnose due to its unclear characteristics which are common to other cancer subtypes. |
[19] |
|
NSCLC Subtype |
Percentage (% Over NSCLC Cases) |
Characteristics |
Ref. |
|
Adenocarcinoma |
40 |
Presents frequent histologic heterogeneity. Mainly affects the outer edges of the lung. |
[10,19] |
|
Squamous cell carcinoma |
30 |
Centrally located in the larger bronchi of the lung. The incidence is linked with smoking more than for other NSCLC cancers. |
[10,19] |
|
Large cell carcinoma |
10 |
Non-differentiated type of lung cancer that lacks the architecture of squamous or glandular differentiation. It usually affects the peripherical part of the lung. |
[10,19] |
|
Adenosquamous carcinoma |
<5 |
It is a subtype presenting components of both adenocarcinoma and squamous cell carcinoma. |
[10,19,20] |
|
Sarcomatoid carcinoma |
<1 |
Centrally located in the larger bronchi of the lung and the peripherical part of the lung. Hard to diagnose due to its unclear characteristics which are common to other cancer subtypes. |
[10,19] |
Table 2. Staging of NSCLC according to the International Association for the Study of Lung Cancer [21].
|
Stage |
Tumor |
Lymph Node |
Metastasis |
|
Occult carcinoma |
TX |
N0 |
M0 |
|
Stage 0 |
Tis |
N0 |
M0 |
|
Stage IA |
T1mi-c |
N0 |
M0 |
|
Stage IB |
T2a |
N0 |
M0 |
|
Stage IIA |
T2b |
N0–N1 |
M0 |
|
Stage IIB |
T1, T2, T3 |
N0–N1 |
M0 |
|
Stage IIIA |
T1–T4 |
N0–N2 |
M0 |
|
Stage IIIB |
T1a–T4 |
N2–N3 |
M0 |
|
Stage IVA |
Any T |
Any N |
M1a–b |
|
Stage IVB |
Any T |
Any N |
M1c |
Unfortunately, often lung cancer is diagnosed at stage III or IV, or even later, owing to the absence of clinical symptoms in early stages. Therefore, it is necessary to continuously monitor those patients at risk of developing lung cancer. In advanced stages, continuous cough, chest pain, or weight loss appear, considerably reducing the quality and life expectancy of the patient [22][23][12,22,23]. Imaging techniques such as radiographs, CT, MRI, or PET (Table A1) allow to locate the tumor and to subsequently perform a biopsy of the identified area and make a diagnosis. Other techniques such as bronchoscopy, mediastinoscopy, or bronchoalveolar lung fluid analysis by bronchoalveolar lavage (BAL) (Box 1) may be necessary to complement the results [12].
The correct prognosis and characterization of the tumor allows selecting the most effective treatment for the patient. Depending on the stage, histological cell type, and clinical condition, a wide variety of treatments are available. In the case of early diagnosis, surgical removal of the tumor, as well as radiotherapy and chemotherapy, are the chosen treatments [24][12,14,24]. However, despite the good short-term results, conventional treatments show a low long-term response rate due to their lack of specificity on the tumor and high off-target cytotoxicity. When locally administered, they show meaningful systematic exposure and diffuse to other tissues, which decreases their efficacy [25]. Looking for better local retention and safer systemic administration is highly desirable. To this end, a wide variety of nano Drug Delivery Systems (DDS), including liposomes, protein-based nanocarriers, inorganic carriers, and polymer nanoparticles, is being explored (Figure 2). The use of customized nanoparticles addressed to cancer cells is the next-generation approach to improve non-specific conventional therapies [26][27][28][29][30][26–30]. The preferential accumulation of nanomedicines in target tissues is commonly referred to as the Enhanced Permeability and Retention (EPR) effect [31][32][31,32]. EPR relies on the presence of abnormally wide gaps in the blood vessels surrounding a tumor. However, the failure of the passive tumor targeting of nanoparticles has questioned the clinical relevance of this phenomenon. Although a rapid tumor growth in animal models leads to the formation of blood vessels with fenestrations, this effect is not proven in human patients. Thus, researchers are now moving from passively targeted nanoparticles to actively targeted ones, addressing them to specific cell populations, like dendritic cells, in some promising immunotherapies [31][32][31,32]. However, the ability to target nanoparticles to a specific cell population is not clearly improved. Active targeting should consider the interaction of nanoparticles with proteins in the human body. Proteins adhere to nanoparticles’ surface, leading to the well-described protein corona effect and hiding the targeting molecules of the nanoparticles. This difficulty, added to recent findings on internalization mechanisms of nanoparticles in solid tumors, highlights the need to better understand the biodistribution of these carriers in the body [33].
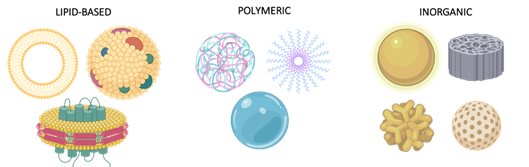
Figure 2. Common Nano Drug Delivery Systems. Nanoparticles can be classified into three main groups: lipidic particles, including liposomes, exosomes, and solid lipid disks among others, polymeric carriers, describing a wide range of vehicles such as polyplexes, micelles, and hydrogels, and inorganic nanosystems, the most heterogenous group, characterized by a variety of components (gold nanoparticles and stars, silica discs and spheres) and applications.
Nanoparticle functionalization, as well as their geometry and materials, largely determine their function and, therefore, the therapeutic outcomes [31]. Furthermore, by modifying these features it is possible to use them as contrast agents for PET or CT imaging techniques, thus developing dual theragnostic platforms [34].
Regardless of the enhancements introduced by nanotools to conventional treatments (Tables 3 and 4), more advanced tumors require combined chemotherapy and radiotherapy as standard of care, in addition to other emerging therapies such as immunotherapy or personalized medicine [35][36][10,32,35,36]. The use of immunotherapy aims to teach a patient's immune system to recognize and eliminate tumor cells, developing a long-term anti-tumor memory of the system [12]. The development of NSCLC vaccines has arisen in recent years, supported by the design of nanoparticles specifically addressed against a cell population, such as dendritic cells [37], to awake a controlled immune response against the tumor. Despite the high expectations, there are still many challenges to be overcome due to the heterogeneity of NSCLC. Differences in overexpressed receptors in NSCLC cells in different patients explain the low percentages of response to this type of therapy [12,36]. Likewise, certain mutations in the genes that encode receptors involved in proliferation and apoptotic mechanisms, such as EFGR, ALK, ROS 1, and those related to the PI3K/Akt/mTOR pathway, can be effectively targeted by drugs inhibiting signaling cascades regulated by these receptors [38][39][40][10,12,32,38–40].
This review aims to discuss different available strategies for the treatment of NSCLC. We will analyze conventional therapies, focusing on the role of chemotherapy and molecular targeted therapies. Furthermore, we will describe emerging immunotherapies, including the design of different vaccines and gene-modulating therapies for the treatment of NSCLC. Lastly, we will provide a complete study of the current therapies for NSCLC and a further understanding of nanomedicine in this field.
2. Emerging Treatments
- Emerging Treatments
Currently, the main treatments for NSCLC remain surgery, chemotherapy, and radiotherapy, but the strong limitations concerning their efficacy over time and their side effects have pushed research to new alternatives. Despite the incorporation of molecularly targeted therapies in combination with chemotherapy, the increase in NSCLC patients’ overall survival (OS) has achieved a plateau effect in the last years [41][42][43][43,102,103]. The use of immunotherapy, together with gene therapy, is expected to stimulate the production of the next generation of drugs, improving the overall responses in NSCLC patients.
2.1. Immunotherapies
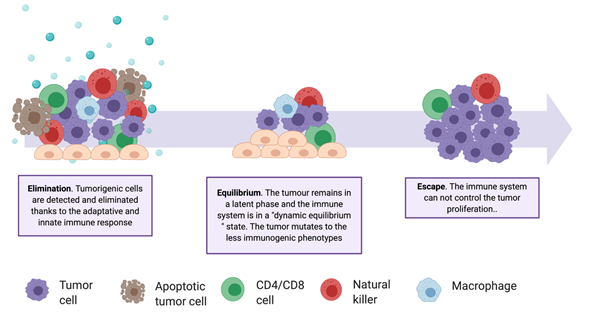
In recent years, immunotherapies have gained importance, as evidenced by the increase of related clinical studies [44][45][104,105]. These treatments stimulate the immune system in multiple ways and are personalized, being based on a patient's genetic and epigenetic alterations. Historically, it has been considered that the immune system does not have, or has very little, response capacity against tumors. However, in 1950, Brunet and Farlane introduced the idea of immunosurveillance, proving that both the innate and the adaptive immune systems are capable of detecting a tumor and reacting to it in the early stages of its development [46][47][48][106–108]. Despite immunosurveillance, tumors continually develop resistance and defense mechanisms, creating a tumor immunosuppressive microenvironment (TIME) and escaping the action of natural killer cells, CD8+ T cells, CD4+ T cells, and macrophages (Table A2). The communication between the two environments—the tumor and the immune environments—is described by the immunoediting theory (Figure 3), which includes three stages: elimination, equilibrium, and escape [48][108]. Immunotherapies are mainly focused on the enhancement of the responsiveness of tumor-infiltrating immune cells during the elimination stage. Several approaches are investigated for this purpose, involving vaccine design, modification of immune cells, and inhibition of tumor evasion mechanisms.
Figure 3. The complexity of the tumor–immune system relationship. Schematic representation of the three phases of the Immunoediting Theory and of the involved cell types. Tumor heterogenicity increases through the elimination phase, selecting the less immunogenic variants.
2.1.1. Immune Checkpoint Inhibitors
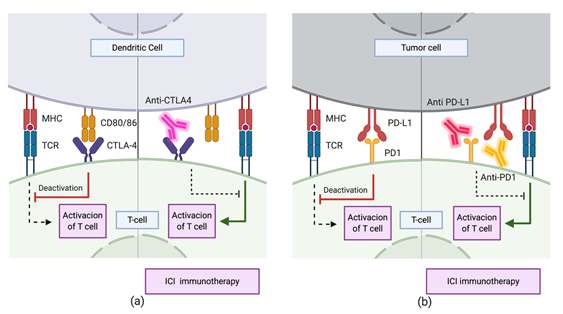
Current immunotherapeutic strategies are mainly based on Immune Checkpoint Inhibitors (ICIs) [49][102,109]. These drugs target routes regulating the activation of the immune system through lymphocytes. The CTLA-4 and PD-1 immune checkpoint pathways have been extensively studied and characterized (Figure 4). Both inhibit T cell activation through different mechanisms and at different levels [50][110]. CTLA-4-dependent mechanism works in the early stages of immune activation, avoiding an overresponse from the immune system. Upon activation of T cells by antigen presentation, CTLA-4 expression occurs on the cell surface. This receptor interacts with CD80 and CD86 expressed on the membrane of Antigen-Presenting Cells (APC). The interaction inhibits the immune response and is enhanced by the tumor microenvironment (TME) through the release of different cytokines. Treatments with CTLA-4 checkpoint inhibitors consist mainly in the administration of antibodies with high recognition specificity for CTLA-4, preventing it from binding to CD80/86 ligands [49][50][109,110]. On the other hand, the PD-1/PD-L1 mechanism downregulates the immune response in late stages, during the effector phase of the innate–specific response of the immune system. The expression of PD-1 in the membrane of natural killer cells and the T/B cells occurs after its activation. The interaction of this protein with its PD-L1 and PD-L2 ligands downregulates the activity of T cells, avoiding autoimmune responses in healthy systems. However, in the TME, tumor cells either express these ligands on their surface or promote their expression by other immune cells through the segregation of factors such as IFN- The TME suppresses the immune response by limiting the activation, proliferation, survival, and effector functions of T cells. Thus, the administration of specific antibodies to PD-1 blocks the interaction of this protein with PD-L1 and PD-L2, avoiding the downregulation of this pathway. Other drugs inhibit it by sequestering PD-L1, which also inhibits the action of T cells. However, as the engagement of PD1 to PD-L2 is still possible, the downregulation of the pathway is limited, and as a result, the former strategy reduces the immune-related toxicity and the side effects of therapy.
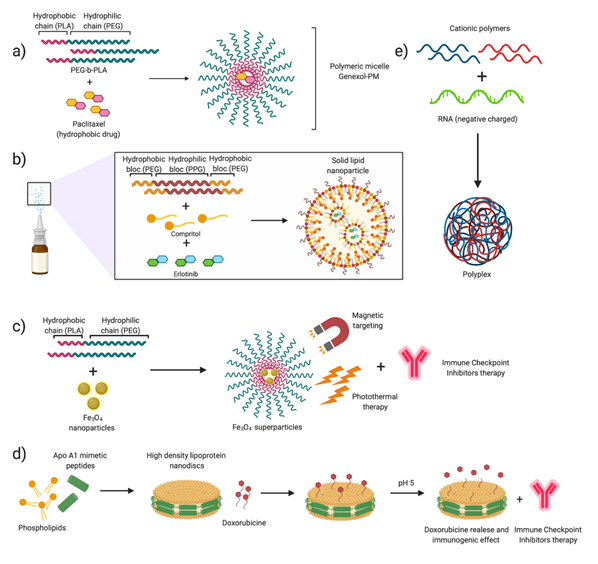
The blockade of the CTLA-4 and PD1/PDL-1 mechanisms consists mainly in the administration of immunoglobulin G (IgG). Different agents are under clinical evaluation, and some of them, such as nivolumab and pembrolizumab, have been approved for the treatment of NSCLC [51][52][111,112]. The principal differences among them lie in the isotype of the administered IgG, as well as in their binding specificity, leading to differences in clinical activity [102]. Numerous clinical studies have evidenced that checkpoint inhibitors-based immunotherapy is effective as a second-line treatment or in advanced-stage patients, mostly in combination with chemotherapy, although pembrolizumab and atezolizumab are also administered as monotherapy [96,99,100] (Table 3). Efforts are currently focused on the performance of head-to-head studies that allow direct comparison between single agents and an agent in combination with chemotherapy [53][113]. Despite their potential, to date, clinical results indicate that ICIs only benefit a subset of patients and present low response rates [54][114]. Importantly, recent studies have evaluated the improvements of ICIs’ anti-tumoral effect when these molecules are combined with chemotherapeutic drugs, which can also modulate the immune activity. Preclinical studies on the combination of nanoparticle-mediated chemotherapy and immune checkpoint inhibitors have shown highly interesting results in murine tumor models. Kuai et al. (2017) have recently published the design and preparation of a chemotherapeutic drug delivery system consisting of high-density synthetic lipoprotein (sHDL) nanodiscs. This type of delivery platform allows the safe and effective release of different chemotherapeutic drugs and enhances the immune response by inhibiting the PD1/PD-L1 pathway [55][56][115,116].
Figure 4. Immune checkpoint inhibitor immunotherapy through mechanisms involving (a) CTLA-4 and (b) PD1/PD-L1. During the priming phase, T cell activation requires two complementary signals: the engagement of the MHC complex to the T cell receptor (TCR) and the absence of interaction between CTLA-4 and CD80/86. Conversely, T cell activation will be strongly suppressed. Similarly, during the effector phase, the absence of interaction between PD1 and PD-L1 upregulates T cell activation.
In addition to the co-administration with chemotherapy, immune checkpoint inhibitors can be combined with drug-loaded nanoparticles [57][32,117]. One of the most promising emerging strategies in this field is the use of photodynamic and thermal nanoparticle-enhanced therapies [58][118]. Photothermal therapies (PTT), used to photosensitize and treat localized cancers, are minimally invasive and are based on the release of vibrational energy from nanomaterials to ablate cancer cells. The advantage of this type of combined therapy compared to checkpoint inhibitors is that they allow overcoming the adaptive immune evasion mechanisms of tumors. Ge et al. (2018) [59][119] proposed the use of iron oxide (Fe3O4 superparticles) based on already FDA-approved particles for magnetic resonance imaging (MRI), encapsulated in spheres of the FDA-approved copolymer mPEG–poly(lactide-co-glycolid) (PLGA). These Fe3O4 superparticles are triggered by near-infrared (NIR) light, producing thermal ablation and killing tumor cells. Besides, these magnetic nanoparticles are encapsulated together with the immune adjuvant Toll-like receptor 7 (TLR7), which stimulates a strong systemic antitumor immune response. This strategy, involving three FDA-approved components, combined with PD-L1 immune checkpoint inhibitors, was proved to directly destroy a tumor upon NIR irradiation and induce dendritic cell activation [59][119].
2.1.2. Therapeutic Vaccines
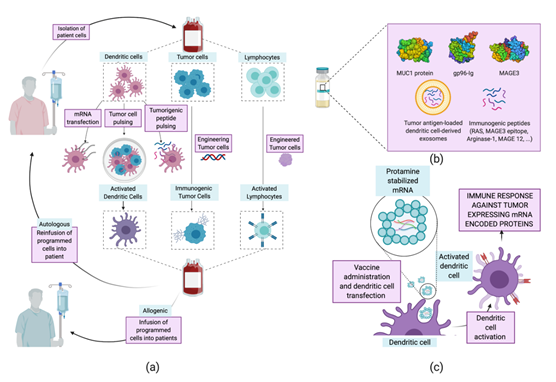
The term “vaccine” has been traditionally related to the treatment of infectious diseases, aiming at humoral immunity against pathogens. These types of vaccine have served as inspiration and have evolved into therapeutic vaccines [60][120]. The latter are designed to treat a disease by boosting the immune humoral and cellular—mainly T cell—response. The concept of a vaccine against cancer arises from the identification of mutated proteins, expressed aberrantly by tumor cells. These are identified as tumor-associated antigens (TAA) by the immune system and can be classified into expressed fetal antigens (normally absent in healthy adults) and overexpressed normal proteins. [60][61][62][120–122] Training the immune system to recognize and respond to these antigens is the working principle of therapeutic vaccines. Several vaccination strategies have been examined for the treatment of NSCLC, including whole-cell vaccines [63][64][65],[123–125], protein- and peptide-based [66][67][126,127] vaccines, and mRNA vaccines [68][69][128,129] (Tables 3 and Figure 5). Herein, we will focus on protein, peptide, and mRNA vaccines, as interesting advances have been achieved regarding their encapsulation using nanoparticles.
Table 3. Relevant clinical trials evaluating cancer vaccines for NSCLC. *N.S.: Not Specified
|
Vaccine |
Components (Brand/Clinical Trial Name) |
NSCLC Stage |
Clinical Study Phase |
Clinical Trial |
|
Cellular vaccine |
Allogenic tumoral cells (1650-G) |
I–II |
II |
NCT00654030, NCT00601796 |
|
Autologous engineered dendritic cells (MIDRIX4-LUNG) |
III |
I |
NCT04082182 |
|
|
Autologous mRNA/DNA transfected dendritic cells (MIDRIXNEO-LUNG) |
III–IV |
I |
NCT04078269 |
|
|
Allogenic mRNA-transfected dendritic cells (AST-VAC2) |
III–IV |
I |
NCT03371485 |
|
|
Allogenic engineered dendritic cells irradiated with seven active agents (NY-ESO-1, MAGE C1, 4MAGE C2, TPGB, Survivn, MUC1, Melan-A antigen (PDC*lung01) |
N.S. |
I–II |
NCT03970746 |
|
|
Autologous dendritic cells pulsed with allogenic tumor cells |
III |
II |
NCT00103116 |
|
|
Allogenic whole tumor cells (Lucanix ®) |
III–IV |
III |
NCT00676507, NCT01058785 |
|
|
Autologous dendritic cells pulsed with allogenic tumor cells (MelCancerVac®) |
III–IV |
II |
NCT00442754
|
|
|
Autologous dendritic cells pulsed with p53 peptide |
III |
II |
NCT00019929
|
|
|
Engineered autologous killed tumor cells |
IV |
I–II |
NCT01159288, NCT02439450 |
|
|
Allogeneic CD4+ memory Th1-like T-cells (Allostim®) |
II–IV |
I–II |
NCT01065441
|
|
|
Autologous dendritic cells pulsed with allogenic tumor cells (DVAC/LuCa) |
IV |
I–II |
NCT02470468
|
|
|
Allogenic lymphocytes |
I–IV |
I |
NCT00161187 |
|
|
Protein vaccine |
MUC1 |
III |
I–II |
NCT01720836, NCT03353675, NCT00415818 NCT03623750 |
|
Heat shock protein (gp96-Ig) |
III–IV |
I |
NCT00503568 |
|
|
Tumor antigen-loaded dendritic cell-derived exosomes |
III–IV |
II |
NCT01159288 |
|
|
Anti-idiotype vaccine |
IIA–III |
II |
NCT00006470 |
|
|
Recombinant PRAME protein |
I–IIIA |
II |
NCT01853878 |
|
|
Peptide vaccine |
IDO peptide |
III–IV |
I |
NCT01219348 |
|
HLA-A*0201 restricted 9-mer epitopes (Vx001) |
IV |
II |
NCT01935154 |
|
|
Short lived proteins (SLiPs) and defective ribosomal products (DRiPs) |
III–IV |
I |
NCT00850785, NCT01909752 |
|
|
Synthetic peptides encoding hTERT (UV1) |
III |
I–II |
NCT01789099 |
|
|
MUC1 peptide (Tecemotide/L-BLP25/Stimuvax®) |
III |
III |
NCT00409188, NCT00960115, NCT00157196, NCT00828009, NCT00157209 |
|
|
UCP2 and UCP4 (telomerase derived peptides) |
III |
I–II |
NCT02818426 |
|
|
Epitope Peptide Restricted to HLA-A*02 |
III–IV |
I |
NCT01069640, NCT01069575 |
|
|
GV1001 (Synthetic peptides encoding hTERT) |
III |
N.E. (already approved in Korea for pancreatic cancer) |
NCT00509457 |
|
|
(MAGE3 epitope) (Astuprotimut-R (GSK-249553)) |
IB–II |
II |
NCT00290355 |
|
|
Wilms tumor 1 (WT1) analog peptide (DSP-7888) |
III–IV |
I |
NCT03715985 |
|
|
Peptides derived from a patient's tumor individual neo-antigens (NeoPepVac, GRT-C901 and GRT-R902, GEN-009, NEO-PV-01) |
III–IV |
I |
NCT03715985, NCT03639714, NCT03794128, NCT03953235, NCT03633110, NCT02897765, NCT03380871 |
|
|
Tedopi® (OSE2101) |
III–IV |
III |
NCT02654587 |
|
|
RAS peptide |
II–IV |
I–II |
NCT00019006, NCT00019331, NCT00003125 |
|
|
Arginase-1 peptide |
Generic |
I |
NCT03689192 |
|
|
YE-NEO-001 Neoepitope yeast vaccine (YE-NEO-001) |
Generic |
I |
NCT03552718 |
|
|
MAGE-12 peptide |
IV |
I |
NCT00020267 |
|
|
Patient specific neoepitopes |
|
|
|
|
|
mRNA vaccine |
NY-ESO-1, MAGE C1, 4MAGE C2, TPGB, Survivn, MUC1 (RNActive®) |
III–IV |
I–II |
NCT03164772, NCT00923312 |
|
KRAS gene vaccine V941 (mRNA-5671) |
III–IV |
I |
NCT03948763 |
|
|
Personalized vaccine against patient’s mutations (RO7198457) |
III–IV |
I |
NCT03289962 |
|
|
DNA vaccine |
NY-ESO-1 plasmid DNA (pPJV7611) to increase immunogenicity of tumor cells |
III–IV |
I–II |
NCT00199849 |
|
Plasmid encoding neoepitopes (VB10.NEO) |
III–IV |
I–II |
NCT03548467 |
Figure 5. Current strategies for NSCLC cancer vaccines. (a) Whole-cell cancer vaccines. Dendritic, tumor, or lymphocyte cells are removed from the patient and modified ex vivo to increase their immune/immunogenic activity. Finally, they are delivered back to the donor patient—autologous therapy—or other patients—allogeneic therapy. (b) Protein- and peptide-based vaccines. Systematic administration of proteins or peptides previously identified as tumor antigens. (c) mRNA vaccines (RNActive®). Administration of five mRNAs, recognized for their immunogenic nature, stabilized by complexing with cationic proteins for the easy transfection of dendritic cells that will activate the immune response, entering the priming phase of T cells.
Protein and Peptide-Based Vaccines.
The use of proteins or peptides for the treatment of cancer is one of the strategies first developed, in parallel with whole tumor cell vaccines. However, two main limitations explain the low response rate of this type of vaccine. First, cancer antigens show low immunogenicity on their own, requiring co-administration of adjuvants to stimulate the immune system [120,121]. Furthermore, the absence of proteins exclusively expressed in cancer cells increases the risk of triggering autoimmune responses. Together with safety issues, antigen proteins present complex glycosylation patterns and are difficult to purify, which hinders the from-bench-to-industry process. Thus, the use of peptides allows improving the stability, selectivity, and reduction of unwanted immune responses concerning the use of the complete protein. Despite this, in recent years several protein vaccines have been consolidated as effective treatments against NSCLC. The melanoma-associated antigen A3 (MAGE-A3) is an antigen almost exclusively expressed in various types of tumor cells. The administration of MAGE-A3 together with immune response-enhancing adjuvants has shown positive results, although not clinically relevant [70][71][72][130]. Similarly, the TG4010 vaccine uses the mucinous glycoprotein-1 protein (MUC1), another well-described tumor-associated protein, as an antigen. Although the vaccine based in the entire MUC1 protein did not obtain notable positive results in various clinical trials [73][131], the use of a 25-aminoacid MUC1 peptide showed remarkable positive outcomes [71][72][132,133]. Encapsulation of peptides in lipid particles allows overcoming limitations regarding peptide stabilization and protection and also improving the uptake by APCs. Furthermore, these types of carrier can be decorated with immune potentiators such as adjuvants or immune cell-targeting ligands [74][116,134].
mRNA Vaccines
mRNA vaccines appeared in the early 1990s after testing the expression of proteins from injected mRna [75][135]. At first, research on vaccines with genetic material focused mainly on DNA. The reason was the high stability of DNA when compared to RNA. However, the appearance of drug delivery nanosystems and the safety of mRNA in terms of mutagenicity and ease of internalization tipped the scale in favor of mRNA [76][134,136]. The activation of the immune response through cellular transfection with mRNA may be obtained through different approaches. Generally, the use of mRNA—encoding for one or more tumor-associated antigens—for the transfection of APCs trains them to recognize the encoded antigens and activate the humoral and cellular immune responses [77][137]. The safety of this type of vaccine and the conclusions obtained so far explain the number of emerging clinical trials (Table 3). RNActive® CV9201 is one of the most promising vaccines available. It is composed of a mixture of five NSCLC-associated antigens which activate the immune response after the extraction of dendritic cells, their transfection, and subsequent delivery to the patient or after the direct administration of mRNA [76][136]. A phase Ib clinical trial of this vaccine proved a detectable immune response in over 65% of the patients. The strength of the induced response was variable, but 48% of the patients showed antigen-specific humoral responses [128]. RNActive® CV9201 is based on the complexation of mRNA with protamine, a cationic protein with a great ability to complex negative molecules and facilitate their cellular internalization. This type of protein-based nanoparticles has shown promising results in in vivo tests, stimulating the adaptive immune response [78] [138]. Nevertheless, it is worth noting that, being an autologous treatment, its application by healthcare systems is not affordable yet. Other studies revealed the promising use of EVs, such as exosomes, in developing cancer vaccines [60]. These vesicles allow both direct release of mRNA-based antigens to induce the immune response and in vitro co-culture of dendritic cells with antigen-loaded exosomes for maturation and subsequent injection into patients [79][139]. Furthermore, these vesicles are engineered through membrane decoration with viral fusion proteins or ligands for Toll-like receptors to enhance the immunogenicity of vaccines [60].
2.2. Modulating Gene Therapy
The main difficulty regarding the development of cancer treatments is the inherent and acquired drug resistance of tumors [80][140]. This remains the biggest obstacle to conventional treatments such as chemotherapy, reducing their short-term efficacy. Modulating gene therapies have gained interest in recent years to sensitize tumor cells against drugs. One of the most promising approaches is the use of RNA to silence the expression of certain proteins involved in tumor resistance. Transfection of tumor cells with silencing (si)RNA or long-non-coding (lnc)RNA related to tumor abnormalities has shown positive results [81][82][83][141–143]. Notably, delivery of siRNA as a gene knockdown strategy for the inhibition of the expression of certain genes related to apoptosis and cell proliferation has been tested as a sensitizing therapy by our group. One well-studied mechanism is the Target of Rapamycin (mTOR) pathway, which regulates cell proliferation and metabolism through the inhibition of apoptosis [48]. Previous studies have proved that siRNA encapsulation in polyplexes allows high transfection rates due to their highly positive charge. This nano drug delivery system presents an excellent endosomal escape capacity, ensuring the cytosolic delivery of siRNA [84][85][86][37,144–146].
In addition to sensitizing tumor cells to increase treatment efficacy and prevent tumor resistance mechanisms, it is necessary to control the metastatic potential and progression of lung cancer. One of the most relevant processes in NSCLC is epithelial–mesenchymal transition (EMT). EMT refers to the conversion of epithelial cells to mesenchymal cells by losing adhesion and gaining regeneration and differentiation capabilities [87][147]. This mechanism increases the metastatic and evolution potential of tumors in cancer patients. Thus, it is necessary to regulate EMT. The administration of micro (mi)RNA—single-stranded short non-coding RNA—allows regulating gene expression by the binding of miRNA to the end of the 3’ untranslated region (UTR) of the target mRNA [88][148]. As a result, the loss of markers and proteins characteristic of epithelial cells is avoided. Also other types of genetic material also can stop this conversion. For instance, Suresh et al. (2019) [89][149] designed antibody-conjugated gelatin nanoparticles that encapsulate siRNA for the inhibition of AXL expression, a kinase involved in various signaling pathways. In this way, they managed to reduce the activity of mTOR while reducing the expression of EMT proteins and increasing the tumor-suppressive activity of the p53 pathway.
Apart from silencing the expression of specific proteins, genome repair of lung cancer cells has become a popular approach for NSCLC treatment. The application of the CRISPR/Cas9 technology allows gene editing and opens a wide range of possibilities [90][150]. This approach is based on the use of single-guide RNA-directed Cas9. This enzyme cleaves the DNA at the point of interest and allows its sequence to be modified, deleted, or replaced. Consequently, the CRISPR/Cas9 system can be ubiquitously applied to knock out oncogenes and study tumor-suppressor genes and resistance-related genes [91][151].
3. Conclusions
- Conclusions
In summary, the number of available treatments for NSCLC continues to expand, driven by the improvements introduced by nanotechnology. Conventional therapies have improved in terms of toxicity and clinical outcomes, thanks to their combination with nanomedicine. Nanodelivery systems aim to concentrate drugs in relevant cell populations and control drug release, improving their long-term effects. Furthermore, emerging immunotherapies show promising results for cancer treatment in the early stages. Novel therapeutic treatments based on siRNA, mRNA, and gene editing are the most encouraging cancer strategies. The perfect integration of these therapies with the versatility of nanotechnology (Figure 6) and their parallel growth suggest that the impact of these treatments on patient lives will be evident in a non-distant future. However, nanotechnology has to develop further. Our limited knowledge about the interactions between nanoparticles and biomolecules makes it difficult to fully understand the mechanisms involved and, therefore, to improve treatment design. Furthermore, research is required to describe the phenomenon of tumor nanoparticle permeability, as the current paradigm regarding EPR is being strongly questioned.
Figure 6. Nanotechnology for NSCLC. Summary of the mentioned examples describing different encapsulation systems. (a) Poly(ethylene glycol)–b-poly(lactic acid) (PLA–b-PEG) nanoparticles encapsulating Paclitaxel (Genexol-PM®) (NCT01023347, NCT01770795). (b) Solid–lipid nanoparticle formation for Paclitaxel encapsulation [99]. (c) Supernanoparticles for photothermal therapy in combination with Immune Checkpoint Inhibitors (ICIs) immunotherapy [119]. (d) Assembly of high-density-lipoprotein nanodiscs for doxorubicin delivery [115]. (e) Encapsulation of RNA in polyplexes, formed by cationic polymers [84][85][86][144–146].