FN-III proteins are widely distributed in mammals and are usually involved in cellular growth, differentiation, and adhesion. The FNDC5/irisin regulates energy metabolism and is present in different tissues (liver, brain, etc.). In large mammals, the regulation of energy homeostasis under metabolic shifts is the foremost challenge to keep normal physiological and molecular functioning. Fibronectin type III domain containing 5 (FNDC5) was initially designated as a critical factor that causes cellular differentiation of skeletal muscle. Principally, it was detected in peroxisomes. Irisin is a myokine involved in higher energy expenditure through stimulation of white adipose tissues. Keeping in view the physiological roles of FN-III proteins (particularly FNDC-5), it is imperative to characterize these proteins at the genomic level to better understand their structure and putative functions in the buffalo.
- buffalo
- FN-III proteins
- FNDC5/irisin
- receptors
- binding affinities
1. Introduction
2. Identification of FN-III Gene Family and Their Physiochemical Properties
| Gene | Chr. | Exon Count | MW (Da) |
A.A | pI | AI | II | GRAVY |
|---|---|---|---|---|---|---|---|---|
| Fibronectin 1 (FN1) | 2 | 46 | 258,641.53 | 2354 | 5.28 | 69.74 | 40.09 | −0.487 |
| Fibronectin type III domain containing 5 (FNDC5) | 2 | 6 | 22,869.33 | 205 | 6.44 | 92.68 | 52.30 | −0.218 |
| Fibronectin type III domain containing 3B (FNDC3B) | 1 | 31 | 127,736.34 | 1160 | 5.91 | 69.91 | 53.98 | −0.434 |
| Fibronectin type III and ankyrin repeat domains 1 (FANK1) | 23 | 14 | 38,413.93 | 345 | 8.51 | 89.51 | 33.76 | −0.334 |
| Fibronectin type III and SPRY domain containing 1 like (FSD1L) | 3 | 16 | 58,607.09 | 521 | 6.32 | 75.93 | 46.15 | −0.574 |
| Leucine-rich repeat and fibronectin type III domain containing 1 (LRFN1) | 18 | 8 | 82,023.66 | 770 | 7.89 | 90.16 | 49.73 | −0.066 |
| Leucine-rich repeat and fibronectin type III domain containing 5 (LRFN5) | 20 | 8 | 52,122.68 | 466 | 6.60 | 95.47 | 35.44 | −0.141 |
| Fibronectin type III and SPRY domain containing 1 (FSD1) | 9 | 13 | 55,768.58 | 662 | 4.96 | 77.88 | 48.72 | −0.380 |
| Fibronectin type III domain containing 3A (FNDC3A) | 13 | 31 | 133,632.56 | 1217 | 6.44 | 71.27 | 46.88 | −0.412 |
| Fibronectin type III domain containing 1 (FNDC1) | 10 | 23 | 205,865.78 | 1905 | 9.66 | 59.01 | 59.92 | −0.799 |
| Leucine-rich repeat and fibronectin type III domain containing 3 (LRFN3) | 18 | 5 | 72,450.76 | 679 | 9.38 | 87.05 | 59.78 | −0.246 |
| Fibronectin type III and SPRY domain containing 2 (FSD2) | 20 | 15 | 84,755.73 | 747 | 4.81 | 69.69 | 47.20 | −0.593 |
| Fibronectin type III domain containing 7 (FNDC7) | 6 | 13 | 85,949.11 | 811 | 6.53 | 77.69 | 45.18 | 0.046 |
| Ankyrin repeat and fibronectin type III domain containing 1 (ANKFN1) | 3 | 20 | 120,567.79 | 1068 | 6.52 | 80.73 | 58.15 | −0.467 |
| Immunoglobulin like and fibronectin type III domain containing 1 (IGFN1) | 5 | 26 | 347,525.99 | 3490 | 6.49 | 55.35 | 34.98 | −0.590 |
| Fibronectin type III domain containing 4 (FNDC4) | 12 | 7 | 24,753.16 | 230 | 7.66 | 88.87 | 55.08 | −0.252 |
| Fibronectin type III domain containing 8 (FNDC8) | 3 | 4 | 34,298.93 | 312 | 5.29 | 80.93 | 46.44 | −0.370 |
| Leucine-rich repeat and fibronectin type III domain containing 4 (LRFN4) | 5 | 3 | 66,839.10 | 636 | 6.70 | 94.14 | 42.55 | −0.028 |
| Fibronectin type III domain containing protein 3C1-like (LOC102393884) | X | 27 | 157,320.54 | 1433 | 6.79 | 71.84 | 45.92 | −0.439 |
| Fibronectin leucine-rich transmembrane protein 2 (FLRT2) | 11 | 4 | 73,773.40 | 660 | 7.89 | 94.18 | 36.58 | −0.185 |
| EGF like, fibronectin type III and laminin G domains (EGFLAM) | 19 | 23 | 112,751.54 | 1032 | 6.53 | 74.46 | 41.70 | −0.325 |
| Fibronectin type III domain containing 9 (FNDC9) | 9 | 2 | 25,342.98 | 227 | 5.65 | 85.99 | 54.56 | −0.055 |
| Leucine-rich repeat and fibronectin type III domain containing 2 (LRFN2) | ||||||||
| Fibronectin leucine-rich transmembrane protein 3 (FLRT3) | 14 | 3 | 73,171.75 | 649 | 7.56 | 94.18 | 44.53 | −0.296 |
| Fibronectin leucine-rich transmembrane protein 1 (FLRT1) | 5 | 2 | 74,144.68 | 677 | 6.15 | 96.88 | 32.12 | −0.122 |
| Fibronectin type III domain containing 11 (FNDC11) | 14 | 4 | 38,198.37 | 333 | 6.81 | 96.34 | 53.23 | −0.280 |
| Fibronectin type III domain containing 10 (FNDC10) |
5 | 3 | 24,097.32 | 225 | 9.11 | 87.33 | 66.15 | 0.124 |
| Extracellular leucine-rich repeat and fibronectin type III domain containing 2 (ELFN2) | 4 | 4 | 90,363.67 | 824 | 7.78 | 81.78 | 48.76 | −0.295 |
| Extracellular leucine-rich repeat and fibronectin type III domain containing 1 (ELFN1) | 24 | 3 | 87,687.60 |
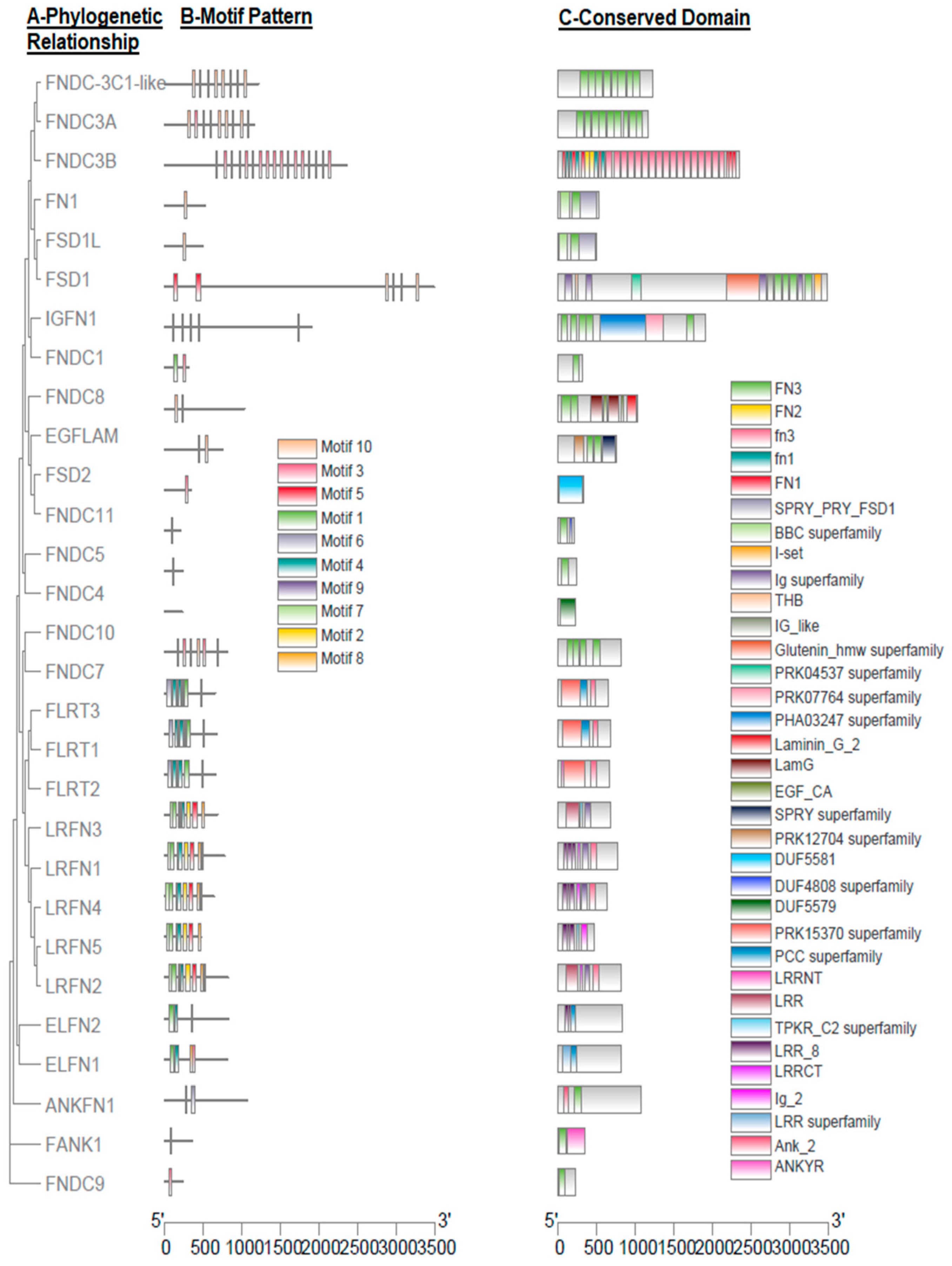
| Motif | Protein Sequence | Length | Pfam Domain |
|---|---|---|---|
| 808 | |||
| 8.82 | |||
| 79.43 | |||
| 61.89 | |||
| −0.351 | |||
3. Gene Structure and Motif Analysis
| MEME-1 | DNFIAAIPRRDFANMTGLVDLTLSRNTISHIEAGAFDDLENLRALHLDNN | 50 | LRR_8 |
| MEME-2 | NPLHCNCELLWLRRLAREDDLETCASPPGLTGRYFWSVPEEEFLCEPPLI | 50 | LRRCT |
| MEME-3 | LTNLEPDTTYRLCVQALNSAG | 21 | fn3 |
| MEME-4 | MVNLETLRLDHNLIDTIPPGAFSELHKLARLDLTSNRLQKL | 41 | LRR_8 |
| MEME-5 | HWVAPDGRLVGNSSRTRVYPNGTLDILITTSGDSGAFTCIASNAAGEATA | 50 | I-set |
| MEME-6 | CPSVCRCDRGFIYCNDRGLTSIPAGIPEDATTLYLQNNQINNAGIPADLK | 50 | LRRNT |
| MEME-7 | CPKRCICQNLSPSLSTLCAKKGLLFVPPNIDRRTVELRL | 39 | Toxin_11 |
| MEME-8 | WPVQRPAPGIRMYQIQYNSSADDTLVYRM | 29 | - |
| MEME-9 | LEDLDLSYNNLESIPW | 16 | LRR_4 |
| MEME-10 | GTEYRFRVRACNEAGEGPLSEPYTVTTPP | 29 | fn3 |
| 2 | |||
| 2 | |||
| 87,694.08 | |||
| 820 | |||
| 6.59 | |||
| 90.88 | |||
| 44.73 | −0.097 |
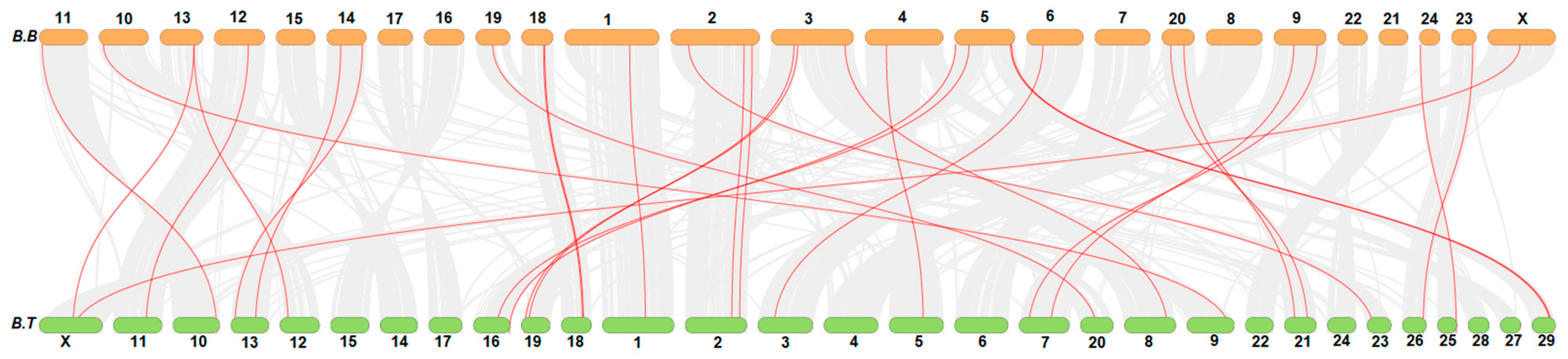
4. Collinearity Analysis of FN-III Gene Family
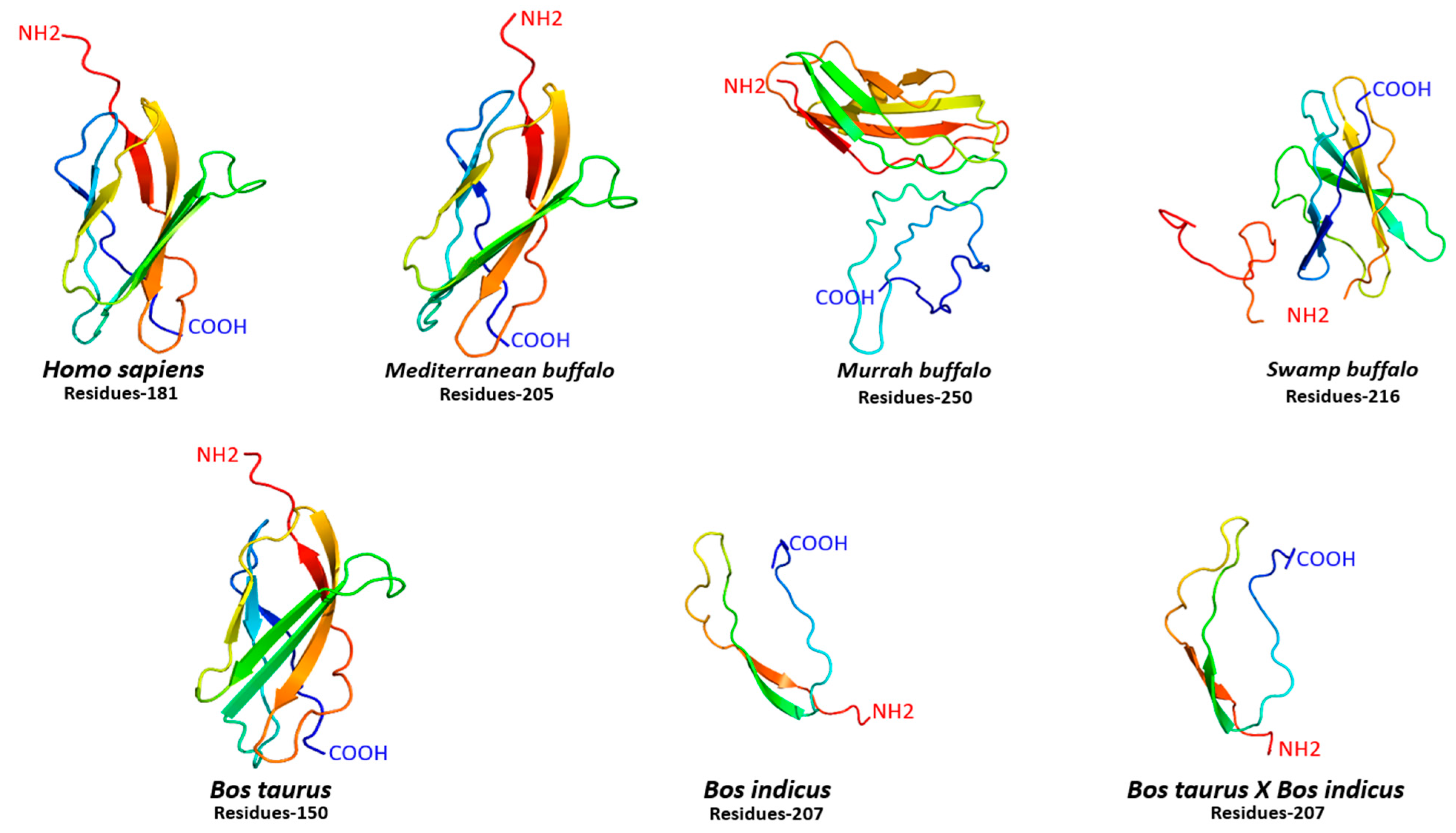
5. Structural Configuration of FNDC5 Protein
6. Multiple Sequence Alignment Analysis of Irisin

7. Molecular Docking Analysis of FNDC5/Irisin
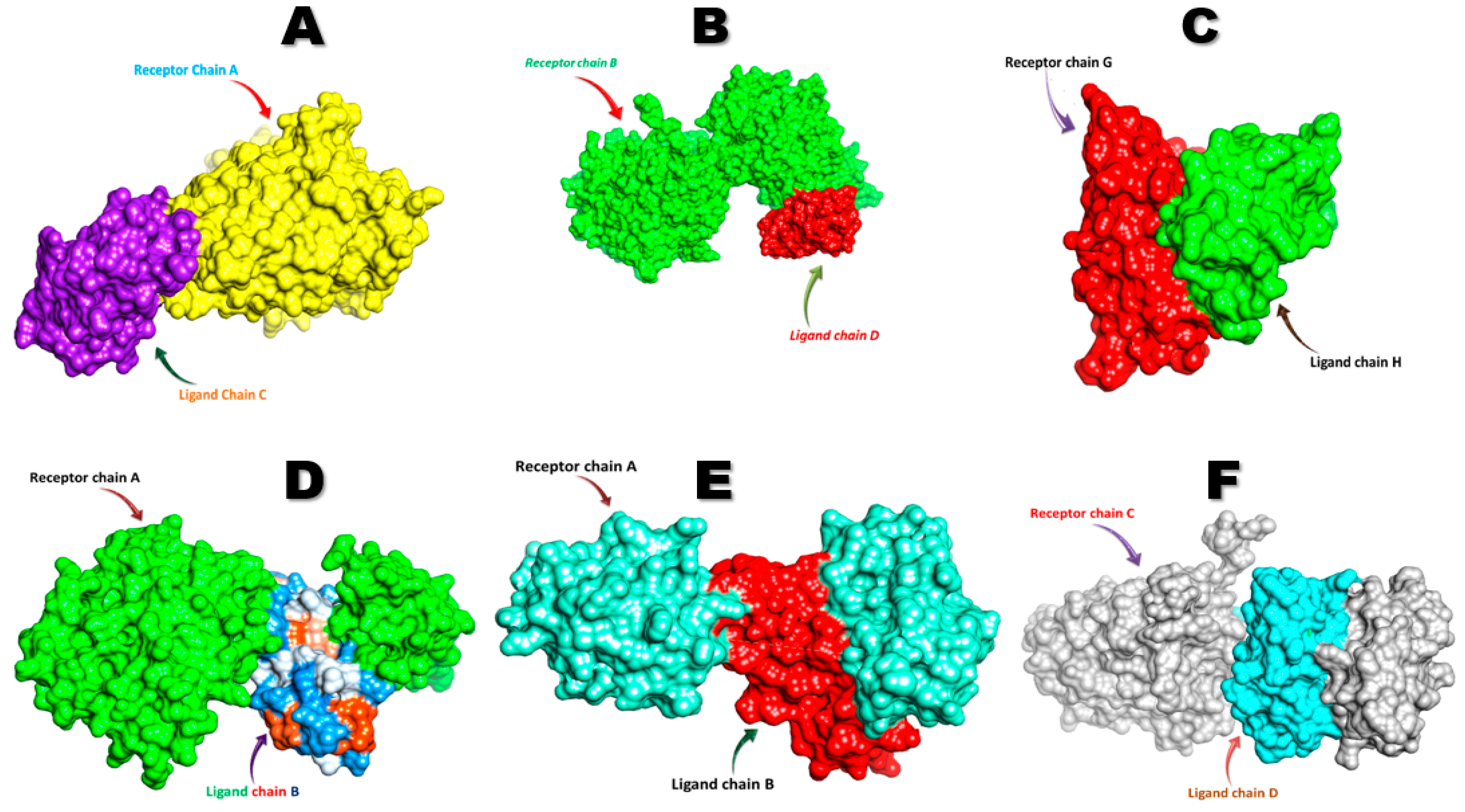
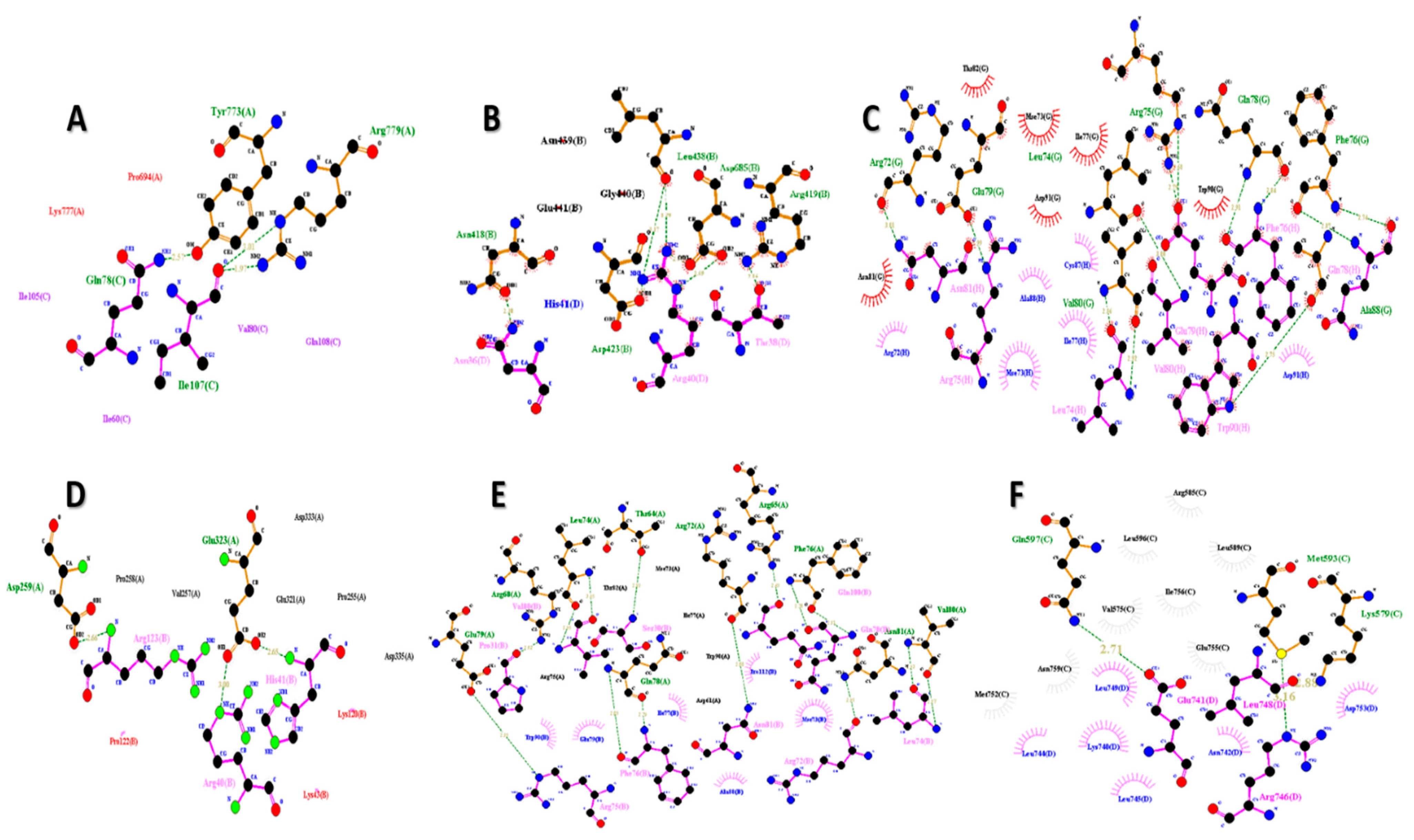
| Sr. No. | Receptor | Docking Score |
Ligand RMSD (A0) |
Ligand Interacting Residues |
|---|---|---|---|---|
| 1 | Androgen | −311.40 | 86.42 | Asn36, Thr38, Arg40 |
| 2 | DDB1 and CUL4 associated factor 6 | −256.63 | 79.76 | Asn36, Thr38, Arg40, His41 |
| 3 | Estrogen-related receptor β | −295.57 | 108.96 | Arg72, Mse73, Leu74, Arg75, Phe76, Ile77, Gln78, Glu79, Val80, Asn81, Cys87, Ala88, Trp90, Asp91 |
| 4 | Estrogen-related receptor γ | −256.63 | 79.76 | Arg40, His41, Lys43, Lys120, Pro122, Arg123 |
| 5 | Krüppel-like factor 15 | −260.71 | 81.85 | Ser30, Pro31, Arg72, Mse73, Leu74, Arg75, Phe76, Ile77, Gln78, Glu79, Asn81, Ala88, Trp90, Gln108, Pro112, Val180 |
| 6 | Nuclear receptor subfamily 3 group C member 1 | −308.59 | 108.34 | Lys740, Glu741, Asn742, Leu744, Leu745, Arg746, Leu748, Leu749, Asp753 |
References
- Pasha, T.; Hayat, Z. Present situation and future perspective of buffalo production in Asia. J. Anim. Plant Sci. 2012, 22, 250–256.
- Rehman, S.U.; Shafique, L.; Yousuf, M.R.; Liu, Q.; Ahmed, J.Z.; Riaz, H. Spectrophotometric Calibration and Comparison of Different Semen Evaluation Methods in Nili-Ravi Buffalo Bulls. Pak. Vet. J. 2019, 39, 568–572.
- Imran, S.; Javed, M.; Yaqub, T.; Iqbal, M.; Nadeem, A.; Mukhtar, N.; Maccee, F. Genetic basis of estrous in bovine: A Review. Int. J. Adv. Res. 2014, 2, 962–966.
- Li, Z.; Lu, S.; Cui, K.; Shafique, L.; ur Rehman, S.; Luo, C.; Wang, Z.; Ruan, J.; Qian, Q.; Liu, Q. Fatty acid biosynthesis and transcriptional regulation of Stearoyl-CoA Desaturase 1 (SCD1) in buffalo milk. BMC Genet. 2020, 21, 1–10.
- Rehman, S.u.; Nadeem, A.; Javed, M.; Hassan, F.U.; Luo, X.; Khalid, R.B.; Liu, Q. Genomic Identification, Evolution and Sequence Analysis of the Heat-Shock Protein Gene Family in Buffalo. Genes 2020, 11, 1388.
- Rehman, S.u.; Feng, T.; Wu, S.; Luo, X.; Lei, A.; Luobu, B.; Hassan, F.-U.; Liu, Q. Comparative Genomics, Evolutionary and Gene Regulatory Regions Analysis of Casein Gene Family in Bubalus bubalis. Front. Genet. 2021, 12, 408.
- Rehman, S.; Hassan, F.-u.; Luo, X.; Li, Z.; Liu, Q. Whole-Genome Sequencing and Characterization of Buffalo Genetic Resources: Recent Advances and Future Challenges. Animals 2021, 11, 904.
- Shi, W.; Yuan, X.; Cui, K.; Li, H.; Fu, P.; Rehman, S.-U.; Shi, D.; Liu, Q.; Li, Z. LC-MS/MS Based Metabolomics Reveal Candidate Biomarkers and Metabolic Changes in Different Buffalo Species. Animals 2021, 11, 560.
- De Rensis, F.; Lopez-Gatius, F. Protocols for synchronizing estrus and ovulation in buffalo (Bubalus bubalis): A review. Theriogenology 2007, 67, 209–216.
- Crowe, M.A.; Mullen, M.P. Regulation and Function of Gonadotropins throughout the Bovine Oestrous Cycle. In Crowe and Mullen; InTech: London, UK, 2013; Volume 143.
- Hassan, F.; Khan, M.; Rehman, M.; Sarwar, M.; Bhatti, S. Seasonality of calving in Nili-Ravi buffaloes, purebred Sahiwal and crossbred cattle in Pakistan. Ital. J. Anim. Sci. 2007, 6, 1298–1301.
- Perera, B. Reproductive cycles of buffalo. Anim. Reprod. Sci. 2011, 124, 194–199.
- Hussain Shah, S.N. Prolonged calving intervals in the Nili Ravi buffalo. Ital. J. Anim. Sci. 2007, 6, 694–696.
- Warriach, H.; McGill, D.; Bush, R.; Wynn, P.; Chohan, K. A review of recent developments in buffalo reproduction—A review. Asian-Australasian. J. Anim. Sci. 2015, 28, 451.
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863.
- Valenick, L.V.; Hsia, H.C.; Schwarzbauer, J.E. Fibronectin fragmentation promotes α4β1 integrin-mediated contraction of a fibrin–fibronectin provisional matrix. Exp. Cell Res. 2005, 309, 48–55.
- Wagenmakers, A.J.; Hawley, J.A.; Hargreaves, M.; Zierath, J.R. Signalling mechanisms in skeletal muscle: Role in substrate selection and muscle adaptation. Essays Biochem. 2006, 42, 1–12.
- Timmons, J.A.; Baar, K.; Davidsen, P.K.; Atherton, P.J. Is irisin a human exercise gene? Nature 2012, 488, E9–E10.
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337.
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778.
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563.
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, İ.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 2014, 61, 130–136.
