Coffee belongs to the genus Coffea of the family Rubiaceae and consists of over 100 species. The two main cultivated species, Coffea arabica L. (Arabica) and C. canephora Pierre ex A. Froehner (Robusta) accounted in 2020, on average, for about 60% and 40% of the world’s coffee production, respectively. Coffee leaf rust (CLR), caused by the biotrophic fungus Hemileia vastatrix, is the most important disease affecting Arabica coffee growing worldwide, leading to significant yield losses if no control measures are applied. A deep understanding of the complex mechanisms involved in coffee-H. vastatrix interactions, such as the pathogen variability and the mechanisms governing plant resistance and susceptibility, is required to breed efficiently for durable resistance and design new approaches for crop protection.
- coffee leaf rust
- rust variability
- effectors
- resistance
1. Introduction


2. Coffee Leaf Rust (CLR) Causal Agent: Life Cycle and Biotrophic Infection Process
3. Rust Variability
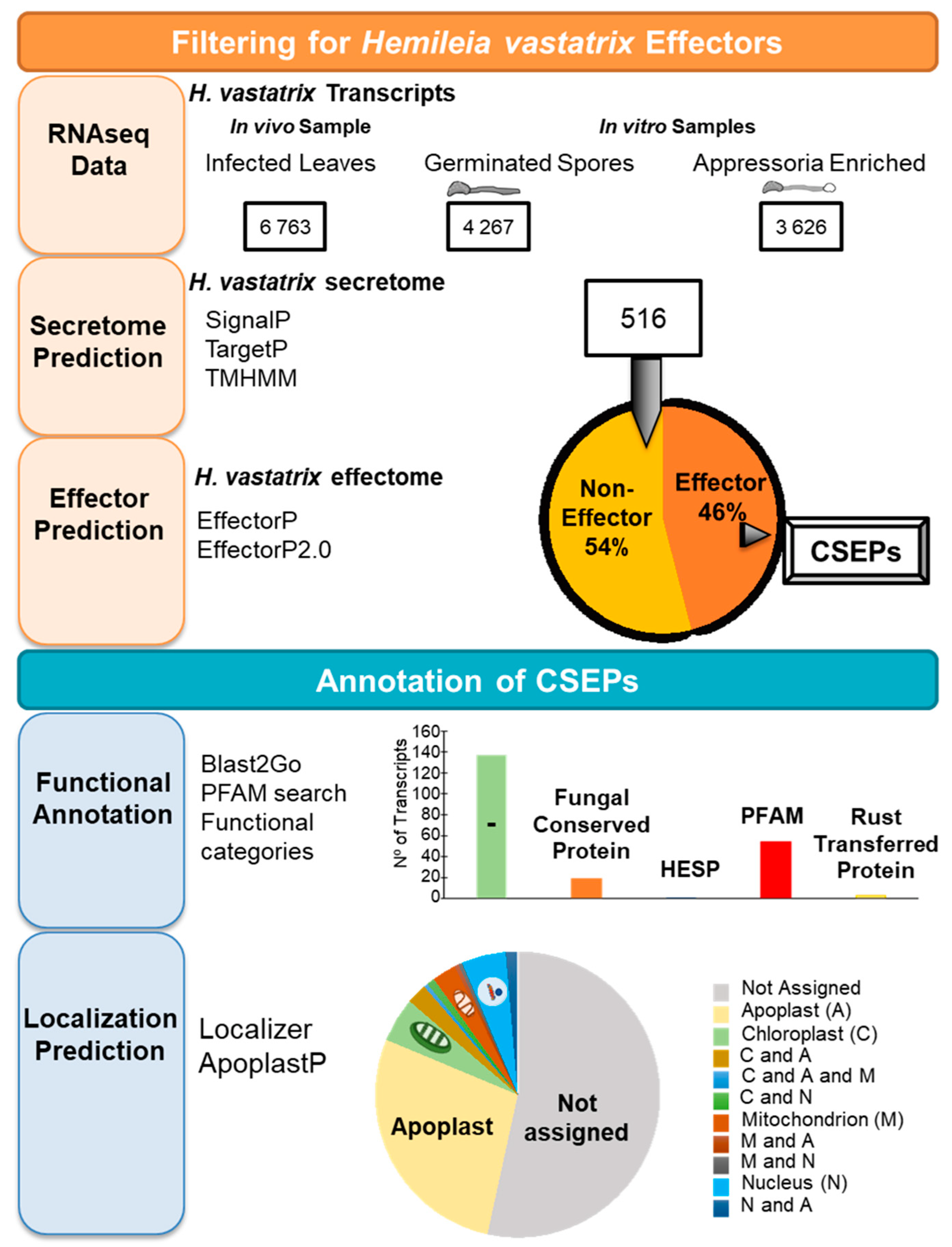
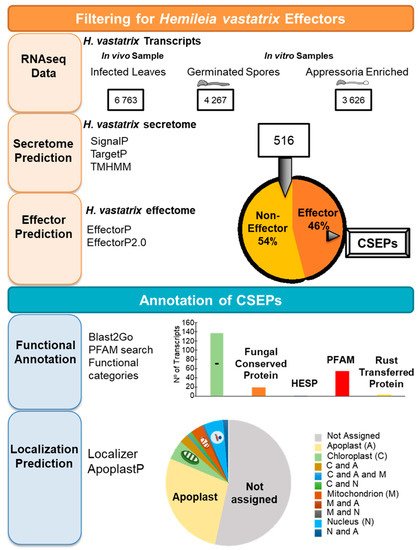
4. Mechanisms of Pathogenicity: The Search for H. vastatrix Effectors
References
- International Coffee Organization (ICO). Coffee Development Report 2019 Overview; International Coffee Organization: London, UK, 2019.
- Davis, A.P.; Tosh, J.; Ruch, N.; Fay, M. Growing coffee: Psilanthus (Rubiaceae) subsumed on the basis of plastid and nuclear DNA sequences; implication for the size, morphology, distribution and evolutionary history of Coffea. Bot. J. Linn. Soc. 2011, 167, 357–377.
- Charrier, A.; Berthaud, J. Botanical classification of coffee. In Coffee: Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Wilson, K.C., Eds.; Croom Helm: London, UK; Sydney, Australia, 1985; ISBN 978-1-4615-6659-5.
- International Coffee Organization (ICO). ICO Coffee Production 2020. Available online: https://www.ico.org/prices/po-production.pdf (accessed on 29 December 2021).
- Davis, A.P.; Chadburn, H.; Moat, J.; O’Sullivan, R.; Hargreaves, S.; Lughadha, E.N. High extinction risk for wild coffee species and implications for coffee sector sustainability. Sci. Adv. 2019, 5, eaav3473.
- van der Vossen, H.; Bertrand, B.; Charrier, A. Next generation variety development for sustainable production of arabica coffee (Coffea arabica L.): A review. Euphytica 2015, 204, 243–256.
- Talhinhas, P.; Batista, D.; Diniz, I.; Vieira, A.; Silva, D.N.; Loureiro, A.; Tavares, S.; Pereira, A.P.; Azinheira, H.G.; Guerra-Guimarães, L.; et al. Pathogen profile—The coffee leaf rust pathogen Hemileia vastatrix: One and a half centuries around the tropics. Mol. Plant Pathol. 2017, 18, 1039–1051.
- Avelino, J.; Allinne, C.; Cerda, R.; Willocquet, L.; Savary, S. Multiple-disease system in coffee: From crop loss assessment to sustainable management. Annu. Rev. Phytopathol. 2018, 56, 611–635.
- Maghuly, F.; Jankowicz-Cieslak, J.; Bado, S. Improving coffee species for pathogen resistance. CAB Rev. 2020, 15, 1–18.
- Kahn, L.H. Quantitative framework for coffee leaf rust (Hemileia vastatrix), production and futures. Int. J. Agric. Ext. 2019, 7, 77–87.
- Bettencourt, A.J.; Rodrigues, C.J. Principles and practice of coffee breeding for resistance to rust and other diseases. In Coffee Agronomy; Clarke, R.J., Macrae, R., Eds.; Elsevier Applied Science Publishers LTD: London, UK; New York, NY, USA, 1988; Volume 4, pp. 199–234.
- McCook, S.; Peterson, P.D. The geopolitics of plant pathology: Frederick Wellman, coffee leaf rust, and cold war networks of science. Annu. Rev. Phytopathol. 2020, 58, 181–199.
- Wellman, F. Peligro de introducción de la Hemileia del café a las Américas. Turrialba 1952, 2, 47–50.
- Keith, L.; Sugiyama, L.; Brill, E.; Adams, B.-L.; Fukada, M.; Hoffman, K.; Ocenar, J.; Kawabata, A.; Kong, A.; McKemy, J.; et al. First report of coffee leaf rust caused by Hemileia vastatrix on coffee (Coffea arabica) in Hawaii. Plant Dis. 2021, 34, 2–4.
- Baker, P. The ‘Big Rust’: An update on the coffee leaf rust situation. Coffee Cocoa Int. 2014, 40, 37–39.
- Avelino, J.; Cristancho, M.; Georgiou, S.; Imbach, P.; Aguilar, L.; Bornemann, G.; Läderach, P.; Anzueto, F.; Hruska, A.J.; Morales, C. The coffee rust crises in Colombia and Central America (2008–2013): Impacts, plausible causes and proposed solutions. Food Secur. 2015, 7, 303–321.
- McCook, S.; Vandermeer, J. The Big Rust and the Red Queen: Long-term perspectives on coffee rust research. Phytopathology 2015, 105, 1164–1173.
- Rhiney, K.; Guido, Z.; Knudson, C.; Avelino, J.; Bacon, C.M.; Leclerc, G.; Aime, M.C.; Bebber, D.P. Epidemics and the future of coffee production. Proc. Natl. Acad. Sci. USA 2021, 118, e2023212118.
- Noronha-Wagner, M.; Bettencourt, A.J. Genetic study of the resistance of Coffea spp. to leaf rust. Can. J. Bot. 1967, 45, 2021–2031.
- Rodrigues, C.J.; Bettencourt, A.J.; Rijo, L. Races of the pathogen and resistance to coffee rust. Annu. Rev. Phytopathol. 1975, 13, 49–70.
- Várzea, V.M.P.; Marques, D.V. Population variability of Hemileia vastatrix vs. coffee durable resistance. In Durable Resistance to Coffee Leaf Rust; Zambolim, L., Zambolim, E., Várzea, V.M.P., Eds.; Universidade Federal de Viçosa: Viçosa, Brasil, 2005; pp. 53–74.
- De Almeida, D.P.; Samila, I.; Castro, L.; Antônio, T.; Mendes, D.O.; Alves, D.R. Receptor-Like Kinase (RLK) as a candidate gene conferring resistance to Hemileia vastatrix in coffee. Sci. Agric. 2021, 78, e20200023.
- Bettgenhaeuser, J.; Gilbert, B.; Ayliffe, M.; Moscou, M.J. Nonhost resistance to rust pathogens—A continuation of continua. Front. Plant Sci. 2014, 5, 664.
- Li, P.; Lu, Y.J.; Chen, H.; Day, B. The lifecycle of the plant immune system. Crit. Rev. Plant Sci. 2020, 39, 72–100.
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of plant defense system in response to microbial interactions. Front. Microbiol. 2020, 11, 1298.
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329.
- Upson, J.L.; Zess, E.K.; Białas, A.; Wu, C.H.; Kamoun, S. The coming of age of EvoMPMI: Evolutionary molecular plant–microbe interactions across multiple timescales. Curr. Opin. Plant Biol. 2018, 44, 108–116.
- De Wit, P.J.G.M.; Mehrabi, R.; Van Den Burg, H.A.; Stergiopoulos, I. Fungal effector proteins: Past, present and future: Review. Mol. Plant Pathol. 2009, 10, 735–747.
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351.
- Han, G.Z. Origin and evolution of the plant immune system. New Phytol. 2019, 222, 70–83.
- Flor, H.H. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 1971, 9, 275–297.
- Lang, J.; Colcombet, J. Sustained incompatibility between MAPK signaling and pathogen effectors. Int. J. Mol. Sci. 2020, 21, 7954.
- Ravensdale, M.; Bernoux, M.; Ve, T.; Kobe, B.; Thrall, P.H.; Ellis, J.G.; Dodds, P.N. Intramolecular interaction influences binding of the flax L5 and L6 resistance proteins to their AvrL567 ligands. PLoS Pathog. 2012, 8, e1003004.
- Peng, Y.; Van Wersch, R.; Zhang, Y. Convergent and divergent signaling in PAMP-triggered immunity and effector-triggered immunity. Mol. Plant-Microbe Interact. 2018, 31, 403–409.
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178.
- Heath, M.C. Hypersensitive response-related death. Plant Mol. Biol. 2000, 44, 321–334.
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease resistance mechanisms in plants. Genes 2018, 9, 339.
- Periyannan, S.; Milne, R.J.; Figueroa, M.; Lagudah, E.S.; Dodds, P.N. An overview of genetic rust resistance: From broad to specific mechanisms. PLoS Pathog. 2017, 13, e1006380.
- Martins, D.; Araújo, S.D.; Rubiales, D.; Patto, M.C.V. Legume crops and biotrophic pathogen interactions: A continuous cross-talk of a multilayered array of defense mechanisms. Plants 2020, 9, 1460.
- Metraux, J. Systemic acquired resistance and salicylic acid: Current state of knowledge. Eur. J. Plant Pathol. 2001, 107, 13–18.
- Cavalcanti, F.R.; Resende, M.L.V.; Carvalho, C.P.S.; Silveira, J.A.G.; Oliveira, J.T.A. Induced defence responses and protective effects on tomato against Xanthomonas vesicatoria by an aqueous extract from Solanum lycocarpum infected with Crinipellis perniciosa. Biol. Control 2006, 39, 408–417.
- Balmer, A.; Pastor, V.; Gamir, J.; Flors, V.; Mauch-Mani, B. The “prime-ome”: Towards a holistic approach to priming. Trends Plant Sci. 2015, 20, 443–452.
- Gozzo, F.; Faoro, F. Systemic acquired resistance (50 years after discovery): Moving from the lab to the field. J. Agric. Food Chem. 2013, 61, 12473–12491.
- Heath, M.C. Signalling between pathogenic rust fungi and resistant or susceptible host plants. Ann. Bot. 1997, 80, 713–720.
- Voegele, R.T.; Mendgen, K.W. Nutrient uptake in rust fungi: How sweet is parasitic life? Euphytica 2011, 179, 41–55.
- Catanzariti, A.M.; Dodds, P.N.; Lawrence, G.J.; Ayliffe, M.A.; Ellis, J.G. Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. Plant Cell 2006, 18, 243–256.
- Garnica, D.P.; Nemri, A.; Upadhyaya, N.M.; Rathjen, J.P.; Dodds, P.N. The ins and outs of rust haustoria. PLoS Pathog. 2014, 10, e1004329.
- Mendgen, K.; Voegele, R. Biology of rusts and mechanisms of infection. In Durable Resistance to Coffee Leave Rust; Zambolim, L., Zambolim, E.M., Várzea, V., Eds.; Universidade Federal de Viçosa: Viçosa, Brasil, 2005; pp. 233–248.
- Azinheira, H.G.; Guerra-Guimarães, L.; Silva, M.C.; Várzea, V.; Ricardo, C.P. Esterase activity and adhesion during the early stages of Hemileia vastatrix differentiation. In Proceedings of the 21st International Conference on Coffee Science (ASIC), France, Montpellier, 11–15 September 2006; pp. 1325–1329.
- Silva, M.C.; Nicole, M.; Rijo, L.; Geiger, J.P.; Rodrigues, C.J. Cytochemistry of plant- rust fungus interface during the compatible interaction Coffea arabica (cv. Caturra)-Hemileia vastatrix (race III). Int. J. Plant Sci. 1999, 160, 79–91.
- Silva, M.C.; Várzea, V.; Guerra-Guimarães, L.; Azinheira, H.G.; Fernandez, D.; Petitot, A.S.; Bertrand, B.; Lashermes, P.; Nicole, M. Coffee resistance to the main diseases: Leaf rust and coffee berry disease. Braz. J. Plant Physiol. 2006, 18, 119–147.
- Cramer, P.J.S. Review of literature of coffee research in Indonesia. Inter-Am. Inst. Agric. Sci. Misc. Publ. 1952, 15, 262.
- Narasimhaswamy, R.L. Coffee leaf disease (Hemileia) in India. Indian Coffee 1961, 25, 382–388.
- Mayne, W.W. Physiological specializalion of Hemileia vastatrix. B. & Br. Nature 1932, 129, 510.
- Mayne, W.W. Annual report of the coffee scientific officer 1938–39. Mys. Coffee Exp. Stn. 1939, 19, 1–16.
- Zhang, H.; Li, J.; Zhou, H.; Chen, Z.; Pereira, A.P.; Silva, M.C.; Várzea, V. Arabica coffee production in the Yunnan province of China. In Proceedings of the 24th International Conference on Coffee Science (ASIC), San José, Costa Rica, 12–16 November 2012; pp. 679–684.
- Noppakoonwong, U.; Khomarwut, C.; Hanthewee, M.; Jarintorn, S.; Hassarungsee, S.; Meesook, S.; Daoruang, C.; Nakai, P.; Lertwatanakiat, S.; Satayawut, K.; et al. Research and Development of Arabica Coffee in Thailand. In Proceedings of the 25th International Conference on Coffee Science (ASIC), Armenia, Colombia, 8–13 September 2014; pp. 42–49.
- Gichuru, E.K.; Ithiru, J.M.; Silva, M.C.; Pereira, A.P.; Várzea, V.M.P. Additional physiological races of coffee leaf rust (Hemileia vastatrix) identified in Kenya. Trop. Plant Pathol. 2012, 37, 424–427.
- Oliveira, B.; Bettencourt, A.J.; Rodrigues, C.J.; Rijo, L.; Azinheira, H.; Talhinhas, P.; Silva, M.C.; Várzea, V. Data Records of CIFC’s Research, 1955–2021. Unpublished work.
- Li, L.; Várzea, V.M.P.; Xia, Q.; Xiang, W.; Tang, T.; Zhu, M.; He, C.; Pereira, A.P.; Silva, M.C.; Wu, W.; et al. First report of Hemileia vastatrix (Coffee Leaf Rust) physiological races emergent in coffee germplasm collections in the coffee—Cropping regions of China. Plant Dis. 2021, 105, 4162.
- Tavares, S.; Ramos, A.P.; Pires, A.S.; Azinheira, H.G.; Caldeirinha, P.; Link, T.; Abranches, R.; Silva, M.D.; Voegele, R.T.; Loureiro, J.; et al. Genome size analyses of Pucciniales reveal the largest fungal genomes. Front. Plant Sci. 2014, 5, 422.
- Porto, B.N.; Caixeta, E.T.; Mathioni, S.M.; Vidigal, P.M.P.; Zambolim, L.; Zambolim, E.M.; Donofrio, N.; Polson, S.W.; Maia, T.A.; Chen, C.; et al. Genome sequencing and transcript analysis of Hemileia vastatrix reveal expression dynamics of candidate effectors dependent on host compatibility. PLoS ONE 2019, 14, e0215598.
- Cristancho, M.A.; Botero-Rozo, D.O.; Giraldo, W.; Tabima, J.; Riaño-Pachón, D.M.; Escobar, C.; Rozo, Y.; Rivera, L.F.; Durán, A.; Restrepo, S.; et al. Annotation of a hybrid partial genome of the coffee rust (Hemileia vastatrix) contributes to the gene repertoire catalog of the Pucciniales. Front. Plant Sci. 2014, 5, 594.
- Silva, D.N.; Várzea, V.; Paulo, O.S.; Batista, D. Population genomic footprints of host adaptation, introgression and recombination in coffee leaf rust. Mol. Plant Pathol. 2018, 19, 1742–1753.
- Rodrigues, A.S.; Silva, D.N.; Várzea, V.; Paulo, O.S. Scaled-up genomic data uncovers a higher level of population genetic structuring in Hemileia vastatrix. In Proceedings of the 28th International Conference on Coffee Science (ASIC), Montpellier, France, 28 June–1 July 2021; p. 40.
- Guerra-Guimarães, L.; Pinheiro, C.; Leclercq, C.; Resende, M.L.V.; Jenny, J.; Ricardo, C.P.; Várzea, V. The first insight on the Hemileia vastatrix urediniospores proteome. In Proceedings of the 28th International Conference on Coffee Science (ASIC), Montpellier, France, 28 June–1 July 2021; p. 223.
- Boufleur, T.R.; Massola Júnior, N.S.; Tikami, Í.; Sukno, S.A.; Thon, M.R.; Baroncelli, R. Identification and comparison of Colletotrichum secreted effector candidates reveal two independent lineages pathogenic to soybean. Pathogens 2021, 10, 1520.
- Vleeshouwers, V.G.A.A.; Oliver, R.P. Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol. Plant-Microbe Interact. 2014, 27, 196–206.
- Gorash, A.; Armonienė, R.; Kazan, K. Can effectoromics and loss-of-susceptibility be exploited for improving Fusarium head blight resistance in wheat? Crop J. 2021, 9, 1–16.
- Fernandez, D.; Tisserant, E.; Talhinhas, P.; Azinheira, H.; Vieira, A.; Petitot, A.S.; Loureiro, A.; Poulain, J.; da Silva, C.; Silva, M.C.; et al. 454-pyrosequencing of Coffea arabica leaves infected by the rust fungus Hemileia vastatrix reveals in planta-expressed pathogen-secreted proteins and plant functions in a late compatible plant-rust interaction. Mol. Plant Pathol. 2012, 13, 17–37.
- Talhinhas, P.; Azinheira, H.G.; Vieira, B.; Loureiro, A.; Tavares, S.; Batista, D.; Morin, E.; Petitot, A.-S.; Paulo, O.S.; Poulain, J.; et al. Overview of the functional virulent genome of the coffee leaf rust pathogen Hemileia vastatrix with an emphasis on early stages of infection. Front. Plant Sci. 2014, 5, 88.
- Sperschneider, J.; Gardiner, D.M.; Dodds, P.N.; Tini, F.; Covarelli, L.; Singh, K.B.; Manners, J.M.; Taylor, J.M. EffectorP: Predicting fungal effector proteins from secretomes using machine learning. New Phytol. 2016, 210, 743–761.
- Sperschneider, J.; Dodds, P.N.; Gardiner, D.M.; Singh, K.B.; Taylor, J.M. Improved prediction of fungal effector proteins from secretomes with EffectorP 2.0. Mol. Plant Pathol. 2018, 19, 2094–2110.
- Loureiro, A.; Tavares, S.; Azinheira, H.G.; Talhinhas, P.; Pereira, A.P.; Fernandez, D.; Silva, M.C. Identification and characterisation of Hemileia vastatrix effectors. In Proceedings of the 25th International Conference on Coffee Science (ASIC), Armenia, Colombia, 8–13 September 2014; p. 250.
- Silva, M.C.; Guerra-Guimarães, L.; Azinheira, H.G.; Talhinhas, P.; Tavares, S.; Loureiro, A.; Diniz, I.; Vieira, A.; Pereira, A.P.; Oliveira, H.; et al. Mechanisms involved in coffee—Hemileia vastatrix interactions: Plant and pathogen perspectives. In Proceedings of the 26th International Conference on Coffee Science (ASIC), Kunming, China, 13–19 November 2016; p. 14.
- Tavares, S.; Azinheira, H.G.; Voegele, R.; Thordal-Christensen, H.; Silva, M.; Talhinhas, P. Identification of Hemileia vastatrix candidate effectors reveals new ways of promoting pathogen variability through alternative splicing. In Proceedings of the 28th International Conference on Coffee Science (ASIC), Montpellier, France, 28 June–1 July 2021; p. 42.
- Maia, T.; Badel, J.L.; Marin-Ramirez, G.; Rocha, C.d.M.; Fernandes, M.B.; da Silva, J.C.F.; de Azevedo-Junior, G.M.; Brommonschenkel, S.H. The Hemileia vastatrix effector HvEC-016 suppresses bacterial blight symptoms in coffee genotypes with the SH1 rust resistance gene. New Phytol. 2017, 213, 1315–1329.
