Hop (Humulus lupulus Linnaeus), a perennial plant belonging to the Cannabaceae family, has become a widely grown agricultural plant because it is used for providing bitterness and aroma to beer. Hop originated in Europe and west Asia, and are cultivated in the United States, Germany, Czech Republic, and England. Historically, the flower extracts of hop, commonly known as hops, have been used in traditional medicine for treating human health because of their sedative, anti-inflammatory, antiseptic, and antidiuretic properties. Hops are a dioecious species and unfertilized female inflorescence are commonly called cones (or strobiles). These cones are rich in unique phenolic compounds such as prenylated flavonoids, humulones (α-acids) and lupulones (β-acids).
1. Introduction
Hop (
Humulus lupulus Linnaeus), a perennial plant belonging to the Cannabaceae family, has become a widely grown agricultural plant because it is used for providing bitterness and aroma to beer
[1]. Hop originated in Europe and west Asia, and are cultivated in the United States, Germany, Czech Republic, and England
[2]. Historically, the flower extracts of hop, commonly known as hops, have been used in traditional medicine for treating human health because of their sedative, anti-inflammatory, antiseptic, and antidiuretic properties
[3]. Various therapeutic effects of hop have increased its interest as a potential bioactive source in the pharmaceutical industry. In Korea, until the 1980s, hop was sufficiently cultivated to meet the demand; however, at present, Korea is dependent on imported hops. However, the Korean craft beer market has been growing in recent years, leading to increased interest toward specialty hops that are produced using domestic hop cultivars.
Hops are a dioecious species and unfertilized female inflorescence are commonly called cones (or strobiles). These cones are rich in unique phenolic compounds such as prenylated flavonoids, humulones (α-acids) and lupulones (β-acids)
[3]. The prenylated flavonoids that include xanthohumol and 6/or 8-prenylnaringenin, act as positive modulators of GABA-induced responses in GABA receptors
[4] and have been characterized as cancer chemopreventive agents
[5]. The alpha acids contain main components such as humulone, cohumulone, and adhumulone, wherein pre-/post-humulone are present in low concentrations. The beta acids are one of the main constituents of the hops resin and also consist of five different components such as lupulone, colupulone, adlupulone, and pre-/post-lupulone
[6]. Humulones and lupulones, two of the most abundant phenolic compounds in hops, are considered as major hop antioxidants.
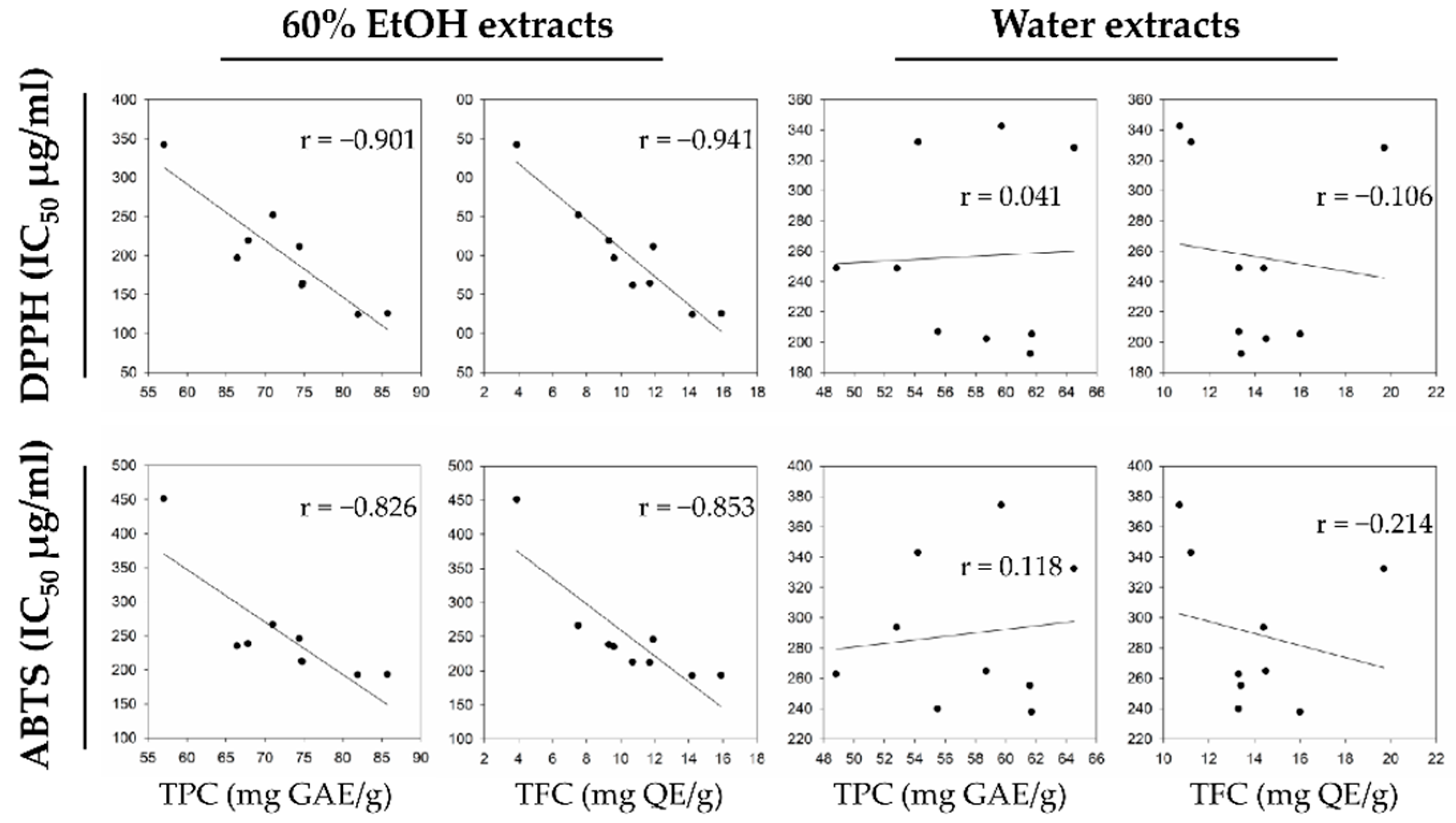
The antioxidant activity of hop or hop extracts was extensively evaluated using different assays, such as the 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) hydroxyl radical scavenging, 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical, and ferric reducing antioxidant power (FRAP) assays
[7][8][9][10]. Previous literatures have consistently reported the correlation between antioxidant activity and different phenolic contents in hop. In particular, the total phenolic content (TPC) and total flavonoid content (TFC) significantly contributed to the antioxidant activity. However, only few hop cultivars or an industrial extract form that includes plug and pellet have been investigated. Recently, new hop cultivars, such as Calypso (released in 2003, USA) and El Dorado (released in 2010, USA) have been released, and research on the antioxidant activity of these cultivars is currently lacking.
2. TPC and TFC Contents
The TPC and TFC were extracted by two different extraction methods, which included 60% ethanol and boiled-water extracts. The efficiency of the phenolic compounds was influenced by several critical factors, such as the extraction solvent, temperature, time, and pH, wherein their impact can either be independent or interactive
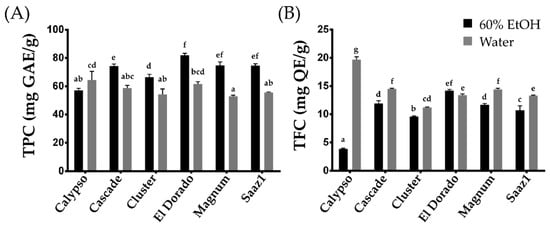
[11]. In the present entry, the levels of TPC extracted by ethanol (TPC_E) and that extracted by water (TPC_W) ranged from 57.00 (Calypso) to 81.90 (El Dorado) mg GAE g
−1, and 52.80 (Magnum) to 64.50 (Calypso) mg GAE g
−1, respectively (
Figure 1A). The TPC levels in water extracts decreased in all cultivars except Calypso, which observed an increase in the levels of TPC. In contrast, under the same extraction methods, the TFC levels ranged from 3.90 (Calypso) to 14.20 (El Dorado) mg QE g
−1 (TFC_E), and from 11.20 (Cluster) to 19.70 (Calypso) mg QE g
−1 (TFC_W) (
Figure 1A). Considering the yield, the TPC content tends to increase in the ethanol extract, whereas the TFC levels increased in the water extracts. In particular, the TFC levels in the water extract of the Calypso cultivar increased approximately four times more than that in the ethanol extract, indicating that the cultivar is rich in water-soluble phenolic compounds. This cultivar can be a valuable genetic resource to further study the changes in TFC characteristics between aqueous and ethanol extracts in hop.
Figure 1. Total Phenolic and total flavonoid content of hop accessions. (A) Total Phenolic content, (B) Total flavonoid content. Letters above each point indicate a significant difference at the 5% level (Tukey HSD tests, n = 3).
A previous study between the aqueous and hydroalcoholic hop extracts also observed similar results for the TPC levels. Kowalczyk et al.
[12] reported that the TPC and TFC levels in hydroalcoholic extracts containing 50% methanol and 50% ethanol were significantly higher than that of the aqueous extract. In researchers' study, the average level of TPC_E at 71.53 mg GAE g
−1 and that of TPC_W at 57.88 mg GAE g
−1 were consistent with the previous results, but the TFC content in both extracts either showed similar levels (El Dorado) or increased levels in water extract.
Additionally, the level of phenolic contents varied depending on their species or cultivars and the environmental condition as described in previous literatures
[13]. The TPC content in hop strobile extracts, widely ranged from 8.7 to 26.5 μg GAE g
−1 [14], from 44.22 to 54.78 mg GAE g
−1 [12], and 887 mg g
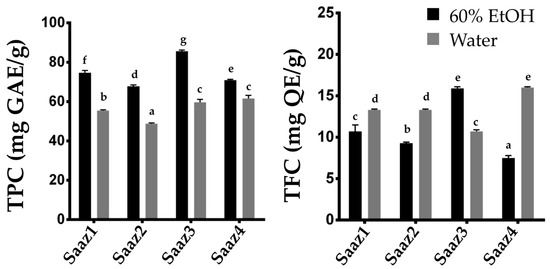
−1 [15]. Therefore, researchers investigated changing levels of phenolic compounds by estimating their content in hops cultivated in different regions (
Figure 2). The Saaz cultivar from Czech, one of the famous hop varieties, is widely cultivated in Korea because of its excellent adaptability to the environmental conditions. As expected, the TPC and TFC levels significantly varied in the ethanol extracts depending on their cultivated regions. Overall, the phenolic compounds in hop strobiles differed depending on the cultivars, extraction solvents, and cultivated regions, which is consistent with the results of previous studies.
Figure 2. Comparison of TPC and TFC contents of Saaz cultivar from different cultivated regions. Total Phenolic content (left), Total flavonoid content (right). The letters above each point indicate a significant difference at the 5% level (Tukey HSD tests, n = 3).
3. Analysis of Prenylflavonoids and Hop Acids
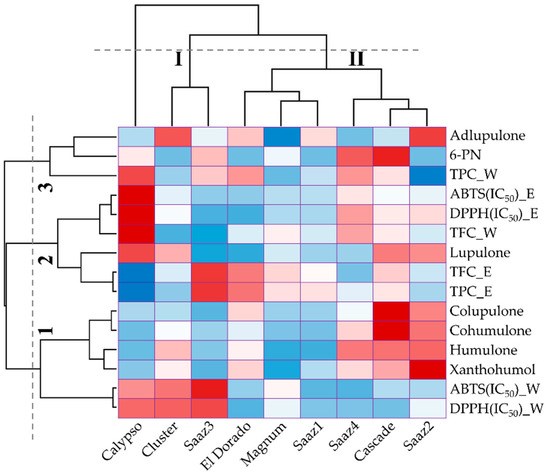
Table 1 shows the identification of prenylflavonoids and hop acids by LC-MS in hop strobile extracts. First, the major hop acids observed in six hop cultivars were identified as: humulones, colupulone, and lupulone, which ranged from 50.44 (Magnum) to 193.25 µg/g (Cascade), from 35.37 (Saaz1) to 73.00 µg/g (Cascade), and from 17.41 (El Dorado) to 52.10 µg/g (Calypso), respectively. In contrast, adlupulone and 6-prenylnaringenin (6-PN) were present in relatively small amounts, especially 6-PN, which remained undetected in the three cultivars Saaz1, Cluster, and El Dorado (
Table 2). Cascade showed higher levels of 6-prenylnaringenin (29.78 µg/g), cohumulone (77.87 µg/g), and colupulone (73.0 µg/g). Although collected from different regions, Saaz2 exhibited higher levels of xanthohumol (53.6 µg/g), humulones (193.25 µg/g), and adlupulone (8.47 µg/g) compared to that of the other cultivars. Humulones and lupulones, which are well-known major flavonoids in hop, are considered as principal bioactive compounds based on their biological and pharmacological properties, such as sedative and cancer chemopreventive activities, antimicrobial activity against bacteria, fungi and viruses, and antioxidant activity
[16][17][18]. Prenylated flavonoids are found in a few families including
Cannabaceae, Moraceae, Rutaceae, and Umbelliferae [19], and these compounds also act in preventing obesity
[20], diabetes
[21], and cancer
[22].
Table 1. Identification of prenylflavonoid compounds by LC-MS.
| No. |
Compound Names |
tR (min) |
Formula |
Molecular Ions (m/z) |
| 415 |
Table 2. Concentration of different prenylflavonoid compounds in hop accessions.
| Cultivars |
6-Prenyl Naringenin |
Xanthohumol |
Cohumulone |
Humulone |
Colupulone |
Lupulone |
Adlupulone |
| 1 |
6-Prenylnaringenin |
35.4 |
C20H20O5 |
| Calypso |
12.42 d |
20.36 e |
8.44 g | 341 |
| 65.48 d |
36.78 d |
52.10 a |
5.36 cd |
2 |
Xanthohumol |
38.4 |
C21H22O5 |
355 |
| Cascade |
29.78 a |
37.88 b |
77.87 a |
188.27 a |
73.00 a |
46.96 ab |
5.55 c |
3 |
Cohumulone |
45.4 |
C20H28O5 |
349 |
| Cluster |
ND |
31.14 c |
29.00 d |
153.31 b |
37.77 d |
41.77 b |
8.21 a |
4 |
Humulone |
46.9 |
C21H30O5 |
363 |
| El Dorado |
ND |
33.81 bc |
25.23 e |
128.09 c |
48.68 c |
17.41 e |
6.88 b |
5 |
Colupulone |
49.3 |
C |
124.25 ± 3.10 a |
192.41 ± 9.55 a25H36O4 |
401 |
| 6 |
Lupulone |
50.5 |
| Magnum |
8.97 e |
15.06 f |
10.17 g |
50.44 e |
35.41 d |
29.40 c |
3.58 e |
C26H38O4 |
415 |
| Saaz1 |
ND |
23.75 d |
9.42 g |
53.84 e |
35.37 d |
24.82 d |
6.60 b |
7 |
Adlupulone |
50.7 |
C26H38O4 |
| Saaz2 |
ND |
53.60 a |
55.87 b |
193.25 a |
57.13 b |
45.13 ab |
8.47 a |
| Saaz3 |
16.35 c |
17.47 f |
14.81 f |
74.43 d |
30.56 e |
16.58 e |
5.93 bc |
| Saaz4 |
24.96 b |
33.33 bc |
38.28 c |
184.68 a |
43.10 c |
24.63 d |
4.78 d |