You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Salvatore Rudilosso.
Lacunar strokes are small subcortical infacts that occur in the territory of one perforatng artery. Lacunar infarcts represent one of the most frequent subtypes of ischemic strokes and may represent the first recognizable manifestation of a progressive disease of the small perforating arteries, capillaries, and venules of the brain, defined as cerebral small vessel disease.
- cerebrovascular disease
- stroke
- ischemic stroke
- lacunar stroke
- small vessel disease
1. Introduction: Clinical Relevance and Aims of the Review
Lacunar ischemic strokes are caused by small infarctions that occur in regions supplied by one perforating artery and represent from 11 to 27% of acute strokes, according to different series [1]. Lacunar strokes have milder symptoms than strokes, due to large vessel disease, and mortality is exceptional during hospitalization. However, about 20% of patients who had a lacunar stroke will present a recurrent cerebrovascular event, 25% will not survive, and 30% will have some degree of functional dependence at five-year follow-up [2]. About half of the patients with a first-ever lacunar ischemic stroke have mild cognitive impairment of subcortical vascular features, and its presence may be a predictor of subcortical vascular dementia in the medium-long-term [3]. Lacunar strokes are not isolated cerebrovascular events, but often represent the tip of the iceberg of a systemic disease affecting the microcirculation, defined as small vessel disease (SVD), which is considered to be the second cause of dementia, as well as the cause of other severe neuropsychiatric disorders, extrapyramidal symptoms, and frailty in the elderly [4]. Despite the high prevalence of lacunar strokes, and the socio-economic impact related to serious long-term-prognostic implications, no specific SVD treatment is available and most of the treatments do not differ from the management of non-cardioembolic ischemic strokes. Notwithstanding, the development of new imaging techniques, or refinement of existing ones, is providing fruitful insights into the diagnosis and pathophysiology of lacunar strokes [5]. Modeling SVD mechanisms and reproducing lacunar strokes in animal models is challenging [6,7][6][7], but translational research remains crucial for identifying new therapeutic targets and developing potential therapeutic agents.
2. Terminology and Correlations between Histopathological, Clinical and Imaging Definitions
The terminology adopted to describe small cerebral infarcts, in the territory of perforating arteries, counts tens of different terms that have been used in research and clinical practice [5]. The discrepancies in the terminology and classification of SVD markers are, in part, the result of the integration of terms deriving from anatomical, histopathological, clinical, and radiological fields, which evolved from the first anatomopathological observation, at the end of the 19th century, to the latest neuroimaging techniques capable of assessing single perforating artery morphology and function (Figure 1).
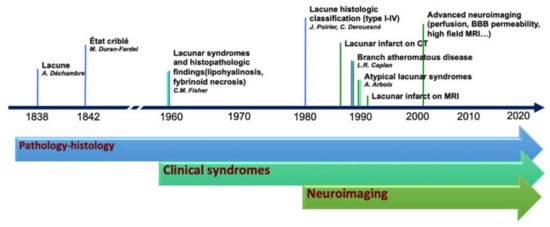

Figure 1. Historical evolution of the knowledge in lacunar strokes.
Lacunar ischemic stroke is a term used to define an acute neurological focal deficit, typically a lacunar syndrome, due to ischemia occurring in a small brain region (<15 mm) supplied by a single perforating artery, consistently with the lacunar hypothesis of an intrinsic small arteriolar disease [8]. From an etiopathological perspective, a lacunar infarct may appear as a small, incomplete infarction in different stages, from early parenchymal rarefaction and variable inflammatory cells and reactive gliosis (type 2a) to complete neuronal loss, spongiosis, and, finally, complete cavitation (type 1a) [9]. The perforating arteries may show typical arteriosclerotic concentric media thickening of the branches smaller than 200 μm, lipohyalinosis, while the more proximal branches (up to 800 μm) may show microatheromas and microvascular thrombosis [8,10][8][10]. On neuroimaging, the lacunar infarcts may correspond to small subcortical lesions with an ischemic appearance, on either CT or MRI, while the small perforating arteries are not visible using conventional imaging acquisitions. It is generally assumed that lacunar syndromes represent the clinical manifestation of a perforating artery’s occlusion, due to progressive lipohyalinosis and superposed microthrombosis, while neuroimaging provides an in vivo surrogate of the small infarction in the brain. Nevertheless, the correlation between clinical syndromes, as well as radiological and histological findings, is not absolute (Figure 2). For example, the identification, on imaging, of an acute cortical stroke necessarily excludes the SVD etiology of the stroke, even if the patient presented with a classical lacunar syndrome. On the other hand, a small subcortical infarct on imaging might be caused by mechanisms other than arteriosclerotic SVD, such as an embolism or large vessel atherosclerotic plaques occluding perforating artery branches. Lacunar infarcts and lacunes may be clinically silent or misrecognized, while disabling lacunar syndromes may be produced by infarcts so small that they might be missed, even when using techniques with high spatial definition, such as an MRI. Histopathological findings include small infarctions in the brain parenchyma, associated with typical lesions of the perforating vessels, such as lipohyalinosis, arteriolar disorganization, and perivascular edema [8,11][8][11]. However, many years might separate the stroke event from the post-mortem study, and clinical–histological correlations might be challenging to establish. Lacunes are small cavities filled by cerebrospinal fluid (CSF) in the subcortical white matter or gray matter structures that might represent the final ischemic stage after a perforating artery occlusion but are also difficult to differentiate from enlarged perivascular spaces in imaging studies.
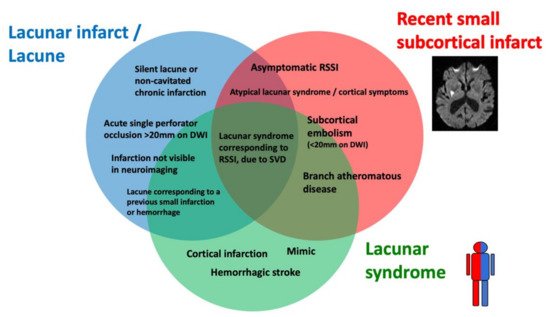

Figure 2. Histological–clinical–radiological correlations in lacunar strokes.
The terminology and definitions used to classify different types of strokes, as well as the different features of SVD, need to be homogenous in research to simplify systematic search strategies and improve the external validity of observational studies and randomized clinical trials (RCTs). Several clinical classifications exist, based on clinical and radiological criteria, to differentiate lacunar strokes from strokes with other etiology. The etiology of stroke subtype may be defined according to different classifications that are based on clinical and radiological criteria. In the Trial of Org 10172 in Acute Stroke Treatment (TOAST) [12], a stroke due to small artery occlusion (lacunar) should present with a traditional lacunar syndrome and have no lesion on imaging, or a subcortical ischemic lesion smaller than 15 mm, in the absence of a mayor cardioembolic source or arterial stenosis >50% on vascular imaging. The atherosclerosis, SVD, cardiac pathology, other causes, dissection classification (ASCOD) [13], and causative classification system for acute ischemic stroke classification (CCS) [14] define the grades of likelihood for SVD etiology. According to the ASCOD definition, the probability of lacunar stroke etiology depends on the presence of a compatible lesion on imaging and other radiological signatures of SVD, such as lacunes or white matter lesions. According to the CCS classification, the probability of lacunar stroke etiology (evident, probable, or possible) is based on the evidence of a consistent lesion on imaging and lacunar syndrome presentation in the absence of alternative causative mechanisms. The standards for reporting vascular changes of neuroimaging (STRIVE) classification was proposed, in order to standardize the different features of SVD in neuroimaging, rather than differentiating lacunar strokes from other strokes subtypes. According to this classification, mostly based on MRI, the result of an occlusion of one perforating artery is classifiable as recent small subcortical infarct (RSSI), white matter hyperintensity (WMH), or lacune, according to clinical–radiological criteria (Figure 3).
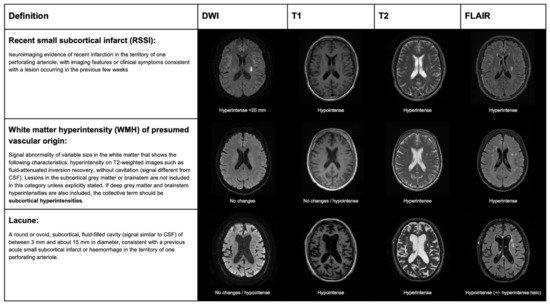

Figure 3. STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) classification for ischemic lesions on MRI, produced by lacunar stroke.
The histopathological features of lacunar infarcts were described by Fisher [8], and a classification for the different appearance of these infarcts, depending on the stage or presence of hemorrhagic features, was proposed by Derouesné and Poirer [9]. However, there is still no consensus for the terminology and reporting of SVD features in histology and their correspondence with imaging features [11,15][11][15].
In this review, we used the term RSSI to refer to neuroimaging evidence, according to the STRIVE definition. We also maintained the term lacunar stroke or syndrome, as appropriated, exclusively to refer to clinical events (i.e., acute management context). In contrast, lacunar infarction (non-cavitated lesion, either acute or chronic) and lacune (chronic cavitated lesion <15 mm) were preferred for pathological findings or assumptions.
3. Mechanisms of Lacunar Strokes: From Pathology Studies to Advanced Neuroimaging
Lacunar ischemic strokes are highly associated with hypertensive arteriosclerosis and other vascular risk factors [16,17,18][16][17][18]. However, about 15–30% of patients with lacunar ischemic strokes had no history of hypertension, suggesting that other vascular risk factors, including aging, and complex mechanisms affecting the microvascular function might play a significant role in the pathogenesis of lacunar stroke. Some of these potential mechanisms that may contribute to perforating artery occlusion, and how they might represent a possible target for therapeutic interventions, are summarized in Table 1.
Table 1. Possible mechanisms involved in lacunar stroke pathogenesis.
| Mechanism | Description | Evidence | Unsolved Issues | Possible Intervention Target | ||||
|---|---|---|---|---|---|---|---|---|
| Hypertensive arteriosclerosis | Progressive hypertensive-related arteriosclerotic injury. Superposed microthrombosis may lead to complete arteriolar occlusion. | Typical histopathological findings in perforating arteries. Indirect evidence from high field MRI techniques [19,20]. | Typical histopathological findings in perforating arteries. Indirect evidence from high field MRI techniques [19][20]. |
Non hypertensive patients may also present with lacunar stroke [21]. In vivo radiological confirmation of small artery wall alterations are not available. | Hypertension is the most modifiable risk factor for stroke secondary prevention [22]. In patients with lacunar strokes, intensive vs. standard blood pressure reduction did not reduce the risk of all stroke recurrency, although it reduced the risk of intracranial hemorrhage (SPS3) [23]. | |||
| Atherosclerosis (branch atheromatous disease) | Atherosclerotic plaques in the main cerebral vessel may occlude the orifice of perforating arterioles [24,25]. | Atherosclerotic plaques in the main cerebral vessel may occlude the orifice of perforating arterioles [24][25]. | Anatomopathological studies [18]. Small plaques are also visible using high field MRI techniques for vessel wall assessment [24]. | Atherosclerosis in large vessel arteries may represent an epiphenomenon. | Lipid lowering is effective for reducing stroke recurrence in non-cardioembolic strokes (SPARCL trial) [26]. Other new drugs aimed to stabilize the inflammatory process in atherosclerosis, which might represent a promising therapeutic target. | |||
| Microembolisms | Small emboli, either from proximal atherosclerotic plaques or cardiac source, may produce single or multiple small subcortical infarcts. | Perforating arteries in lacunar strokes may be patent in pathology studies [8] and advanced 7T MRI techniques [27]. Increased blood flow on CT perfusion suggests recanalization of an embolic occlusion of a perforating artery [28]. Subcortical infarcts in animal models produced by microembolism [6]. | There is an association between atrial fibrillation, load of subcortical infarcts, and WMH [29], but direct evidence of embolism is lacking. Multiple RSSIs do not exclude mechanisms related to SVD (about 20% of RSSI present multiple infarcts, especially in patients with severe SVD [30]). | Treatments aimed to stabilize active plaques or anticoagulant treatment, in case of mayor embolic source. Prothrombotic state (i.e., acute cancer), marantic, or infectious endocarditis should be ruled out in patients with multiple subcortical strokes. | ||||
| Chronic global cerebral hypoperfusion | Chronic hypoperfusion of distal vascular territories may lead to progressive ischemia in the white matter. Small infarctions may occur in the edges of WMH and contribute to SVD progression. | In animal models, small subcortical infarcts may be produced by bilateral carotid occlusions [6]. | The causal relationship between hypoperfusion and SVD progression in longitudinal studies is controversial [31], as hypoperfusion might also be also secondary to reduced metabolism in WMH. | Vasodilatory drugs to increase brain perfusion: mononitrate isosorbide, nitric oxide. (LACI-2) [32]. | ||||
| Inflammation, endothelial dysfunction, and BBB disruption | Endothelial dysfunction may trigger the pro-inflammatory mechanisms promoting pro-thrombotic agents, microglial activation, altered neurovascular homeostasis, and impaired coupling between metabolic demand and nutrient supply. | Markers of BBB leakage in pathology studies [33,34]. Association between the number of lacunes and inflammatory blood markers [35]. BBB permeability on dynamic contrast enhanced MRI is increased in lacunar strokes, compared to cortical strokes [36]. | Markers of BBB leakage in pathology studies [33][34]. Association between the number of lacunes and inflammatory blood markers [35]. BBB permeability on dynamic contrast enhanced MRI is increased in lacunar strokes, compared to cortical strokes [36]. |
Some studies on post-mortem brain samples did not confirm the association of markers of endothelial dysfunction or BBB leakage and SVD [37,Blood markers of endothelial dysfunction and inflammation are are not specific of lacunar stroke subtype [39]. | Some studies on post-mortem brain samples did not confirm the association of markers of endothelial dysfunction or BBB leakage and SVD [ | 38]. A causal relationship with focal BBB leakage prior lacunar strokes is to be determined. BBB permeability variations are mild and difficult to measure in SVD. | 37][38]. A causal relationship with focal BBB leakage prior lacunar strokes is to be determined. BBB permeability variations are mild and difficult to measure in SVD. Blood markers of endothelial dysfunction and inflammation are are not specific of lacunar stroke subtype [39]. |
Anti-inflammatory drugs: colchicine in non-cardioembolic strokes (CONVINCE) [40], uric acid (URICO-ICTUS) [41], and canakinumab [42]. |
| Focal hypoperfusion and compensatory blood flow in acute perforating artery occlusion | Abrupt reduction in blood flow after perforating artery occlusion, regardless the causing mechanisms (either intrinsic SVD or atheroembolic). The extent and the time to establish infarction may depend on factors such as compensatory blood flow through capillary network and cerebrovascular reserve. | Perfusion studies show persistence of residual blood flow, in the territory of perforating arteries corresponding to RSSI [43,44]. Sequential imaging from row perfusion sequences may show retrograde flow, suggesting collateral circulation involvement in RSSI [28,45,46] Microscopic studies showed a dense capillary network, linking contiguous perforating arteries and few arteriolar anastomoses [47]. | Perfusion studies show persistence of residual blood flow, in the territory of perforating arteries corresponding to RSSI [43][44]. Sequential imaging from row perfusion sequences may show retrograde flow, suggesting collateral circulation involvement in RSSI [28][45][46] Microscopic studies showed a dense capillary network, linking contiguous perforating arteries and few arteriolar anastomoses [47]. |
Lack of direct evidence of perforating artery occlusion and recruiting collateral circulation in RSSI | Thrombolysis in lacunar stroke would not be effective without compensatory mechanisms maintaining the tissue viable until recanalization. Perfusion imaging-based thrombolysis, outside of the conventional time window, may also be effective in patients with RSSI. Vasodilatory agents may improve collateral recruitment. Neuroprotective agents may reach the ischemic area through retrograde in the territory supplied by an occluded perforating artery. |
BBB: blood–brain barrier; CONVINCE: colchicine for prevention of vascular inflammation in non-cardioembolic stroke; LACI-2: lacunar intervention trial-2; RSSI: recent small subcortical infarcts; SVD: small vessel disease; SPARCL: stroke prevention by aggressive reduction in cholesterol levels; SPS3: secondary prevention of small subcortical stroke trial; URICO-ICTUS: uric acid in patients with acute stroke trial; WMH: white matter hyperintensities.
Lacunar infarcts are one of the distinctive markers of SVD, including both sporadic [16] and monogenic SVD types (such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) [48,49,50][48][49][50]) and other rare hereditary diseases [51]. In histopathological studies of lacunar infarcts, the perforating arteries typically show thickening of the media, lipohyalinosis, segmental arterial disorganization, and fibrinoid degeneration [18]. However, the histopathological findings represent the end stages of the disease, and the correlations with the clinical event are difficult to establish in most cases. For example, in the pathology studies conducted by Fisher in 114 patients with lacunar infarcts, the perforating arteries supplying the lacunar infarct/lacune had no vascular occlusion, in only 10% of the cases, and only 4 patients had no history of hypertension [8]. A recent systematic review of 39 pathology studies, including more than 4000 lacunar infarcts, highlighted that occlusion of the supplying perforating arteries was found only in a minority of cases, and the prevalence of hypertension was lower, although still higher than 50% of patients [11]. This discordancy could result from therapeutic advances in stroke prevention, such as hypertensive treatment, which could reduce the likelihood of presenting severe progressive stenosis of perforating arteries, while other alternative mechanisms, including embolic occlusions, might have prevailed. However, these mechanistic interpretations are drawn from evidence obtained in post-mortem studies conducted, in most cases months or years after the clinic event, when many factors, including remodeling of the microvasculature, changes in blood flow, and antithrombotic therapies, may have altered the structural features of the small vessels.
Neuroimaging of lacunar stroke has the main advantage of shortening the time from symptoms onset to examination, providing imaging with high temporospatial accuracy and offering insights on the mechanisms occurring during infarction and on the course over time in longitudinal studies. However, imaging evidence has to be considered indirect in most cases, especially in SVD studies, as the small branches of perforating vessel are not visible, not even using high-definition MRI (although the main branches may be visualized using high resolution MRI angiography at 7T [27]). Much information provided by imaging studies is difficult to generalize and depends on many factors, including different criteria to define markers in imaging, acquisition protocols, processing software, and interobserver reliability. Therefore, new markers in imaging research studies need to be tested through various steps of validation. In brief, a newly discovered marker needs a proof of concept (does the marker measure a specific change related to a disease process?) and proof of principle (discriminates cases vs. controls, severity, or prognosis). The technique to assess the marker should be repeatable (precision under the same operating conditions) and reproducible (precision under different operating conditions). Finally, the marker should be effective as an endpoint for clinical studies (i.e., surrogate of a clinical endpoint) and cost-effective for use in large multicentre clinical trials. However, only a few markers in imaging used in SVD research meet most of these validation criteria. More detailed information on the validation of imaging markers in SVD is available in the harmonizing brain imaging methods for vascular contributions to neurodegeneration (HARNESS) position paper [52].
Deep perforating arteries are currently considered end-terminal vascular territories that supply subcortical white matter and deep grey structures. According to this classical theory, the occlusion of a perforating artery would irreversibly lead to infarction of the whole tissue, supplied by an occluded perforating artery within a few minutes after occlusion [53]. However, about 20% of patients with a lacunar stroke presented a transient ischemic attack in the previous hours or days [54], and patients presenting with a lacunar syndrome may recover without showing any ischemic lesion on brain imaging [55]. Perfusion studies on CT and MRI showed that small areas of hypoperfusion (but not a complete absence of it) are visible in some patients with a confirmed RSSI on follow-up imaging, including areas of potentially viable tissue (ischemic penumbra) [43,44][43][44], in contrast with the hypothesis of a complete flow obstruction, without compensation in a terminal arterial territory. The capsular warning syndrome was first described in patients with lacunar stroke involving the internal capsule presenting repeated, stereotyped episodes of motor lacunar syndrome or sensorimotor lacunar syndrome, within 24–72 h, with complete recovery between episodes, which involved two of three body parts (face, arm, or leg), or more, without cortical symptoms [56,57][56][57]. Other studies described lacunar strokes with a similar clinical stuttering course, in other anatomical regions as the pons [58,59][58][59]. Several mechanistic interpretations of the capsular warning syndrome have been formulated, including hemodynamic failure in the presence of a stenotic perforating artery, arteriolar vasospasm, and peri-infarct depolarization [56,60][56][60]. However, the fluctuating insufficiency of a residual blood flow compensation through collateral vessels from nearby perforating arteries would also be consistent with the clinical stuttering presentation. Although perforating artery branches are not directly visible in vivo using conventional imaging, three perfusion studies, one based on CT perfusion [28] and two on MRI perfusion [45,46][45][46], provided similar results of indirect evidence of hemodynamical compensation, through retrograde blood flow filling centripetally the ischemic regions that evolved into a RSSI, suggesting the presence of microscopic collateral supply by a capillary network. In few cases, the ischemic area showed an early and anterograde filling, corresponding to normal or increased perfusion, indicating the patency of the perforating artery during image acquisition, consistent with recanalization, suggesting an embolic origin of the occlusion in a minority of patients [28].
Although small perforating vessels are not directly visible on conventional MRI, small thrombi might be spotted as blooming artifacts on gradient-echo or SWI sequences in the pathway of a perforating artery in less than 20% of patients with a RSSI [61]. However, this technique probably has low sensitivity (no gold standard is available in imaging), and specificity could be hampered by badly impaired blood–brain barrier (BBB) permeability and hemosiderin deposits. Other non-conventional techniques based on high-resolution MRI for vessel wall imaging enable the detection of non-stenotic atherosclerotic plaques occluding emerging perforating arteries, despite apparently normal vascular imaging [19,62][19][62]. The number and morphology of the perforating arteries are also evaluable using high field MRI [63]. However, the long acquisition time and sensitivity to movement artifacts limit the feasibility of these techniques in patients with acute strokes.
In the last 20 years, many authors focused their attention on vascular and endothelial dysfunction in SVD using imaging to assess vascular function measures for BBB permeability, blood flow, and vascular stiffness [4]. The endothelial dysfunction seems to have a crucial role in the pathogenesis of SVD, involving vascular inflammatory mechanisms altering the BBB permeability and extravasation of inflammatory particles into the extracellular space, causing perivascular edema and microglial dysfunction. Endothelial inflammation may increase pro-thrombotic activity, favoring microthrombosis, and affect small vessel autoregulation and capillary heterogeneity [4]. Early studies, assessing BBB permeability using dynamic contrast-enhanced MRI, showed that patients with lacunar strokes have increased BBB permeability, compared to patients with cortical strokes [64]. Other measures of vascular function as cerebrovascular reactivity appeared to be impaired in patients with SVD [65] and lacunar strokes [66] in a few cross-sectional studies. However, it is difficult to obtain these measures prior to the appearance of new subcortical infarcts in longitudinal studies, due to the relatively low rates of incident strokes and use of secondary prevention measures.
The radiological fate of RSSI is variable and hardly predictable, as one-third of RSSI evolve into a lacune, while others may disappear or leave a non-cavitated lesion [67,68][67][68]. BBB leakage into CSF appeared to be a predicting factor of cavitation in one longitudinal study [69], but further longitudinal studies might identify other risk factors. The clinical and prognostic relevance of RSSI cavitation remains to be determined, although some associations have been found with severe progression of SVD and cognitive impairment [70]. Nevertheless, most lacunes might not be related to prior clinical events and represent accidental findings on neuroimaging studies. The spatial correlation with motor and sensitive pathways is highly related to overt clinical symptoms [71], but some lacunes, even in eloquent areas, might not have been recognized clinically. Therefore, some lacunes might result from progressive injury and cavitation, which could be less clinically evident. For example, new cavities usually appear silently in the edges of white matter hyperintensities in patients with CADASIL [72], and lacunes are associated with deep medullary vein stiffening and occlusion, due to venous collagenosis [73].
References
- Arboix, A.; Martí-Vilalta, J.L. New Concepts in Lacunar Stroke Etiology: The Constellation of Small-Vessel Arterial Disease. Cerebrovasc. Dis. 2004, 17 (Suppl. 1), 58–62.
- Norrving, B. Long-Term Prognosis after Lacunar Infarction. Lancet Neurol. 2003, 2, 238–245.
- Blanco-Rojas, L.; Arboix, A.; Canovas, D.; Grau-Olivares, M.; Oliva Morera, J.C.; Parra, O. Cognitive Profile in Patients with a First-Ever Lacunar Infarct with and without Silent Lacunes: A Comparative Study. BMC Neurol. 2013, 13.
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small Vessel Disease: Mechanisms and Clinical Implications. Lancet Neurol. 2019, 18, 684–696.
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging Standards for Research into Small Vessel Disease and Its Contribution to Ageing and Neurodegeneration. Lancet Neurol. 2013, 12, 822–838.
- Bailey, E.L.; Mcculloch, J.; Sudlow, C.; Wardlaw, J.M. Potential Animal Models of Lacunar Stroke: A Systematic Review. Stroke 2009, 40, e451–e458.
- Mustapha, M.; Nassir, C.M.N.C.M.; Aminuddin, N.; Safri, A.A.; Ghazali, M.M. Cerebral Small Vessel Disease (CSVD)—Lessons from the Animal Models. Front. Physiol. 2019, 10, 1317.
- Fisher, C.M. Lacunar Strokes and Infarcts: A Review. Neurology 1982, 32, 871–876.
- Poirier, J.; Derouesne, C. Cerebral Lacunae. A Proposed New Classification. Clin. Neuropathol. 1984, 3, 266.
- Lammie, G.A. Hypertensive Cerebral Small Vessel Disease and Stroke. Brain Pathol. 2002, 12, 358–370.
- Bailey, E.L.; Smith, C.; Sudlow, C.L.M.; Wardlaw, J.M. Pathology of Lacunar Ischemic Stroke in Humans—A Systematic Review. Brain Pathol. 2012, 22, 583–591.
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41.
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Wolf, M.E.; Hennerici, M.G. The ASCOD Phenotyping of Ischemic Stroke (Updated ASCO Phenotyping). Cerebrovasc. Dis. 2013, 36, 1–5.
- Ay, H.; Furie, K.L.; Singhal, A.; Smith, W.S.; Sorensen, A.G.; Koroshetz, W.J. An Evidence-Based Causative Classification System for Acute Ischemic Stroke. Ann. Neurol. 2005, 58, 688–697.
- Humphreys, C.A.; Smith, C.; Wardlaw, J.M. Correlations in Post-Mortem Imaging-Histopathology Studies of Sporadic Human Cerebral Small Vessel Disease: A Systematic Review. Neuropathol. Appl. Neurobiol. 2021.
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of Sporadic Cerebral Small Vessel Disease: Insights from Neuroimaging. Lancet Neurol. 2013, 12, 483–497.
- Regenhardt, R.W.; Das, A.S.; Lo, E.H.; Caplan, L.R. Advances in Understanding the Pathophysiology of Lacunar Stroke: A Review. JAMA Neurol. 2018, 75, 1273–1281.
- Caplan, L.R. Lacunar Infarction and Small Vessel Disease: Pathology and Pathophysiology. J. Stroke 2015, 17, 2–6.
- Xie, W.; Wang, C.; Liu, S.; Tang, R.; Chai, S.; Guo, Y.; Qian, T.; Chang, B.; Yang, Q.; Fan, Z.; et al. Visualization of Lenticulostriate Artery by Intracranial Dark-Blood Vessel Wall Imaging and Its Relationships with Lacunar Infarction in Basal Ganglia: A Retrospective Study. Eur. Radiol. 2021, 31, 5629–5639.
- Ling, C.; Fang, X.; Kong, Q.; Sun, Y.; Wang, B.; Zhuo, Y.; An, J.; Zhang, W.; Wang, Z.; Zhang, Z.; et al. Lenticulostriate Arteries and Basal Ganglia Changes in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy, a High-Field Mri Study. Front. Neurol. 2019, 10, 1–9.
- Arboix, A.; Altés, E.; García-Eroles, L.; Massons, J. Clinical Study of Lacunar Infarcts in Non-Hypertensive Patients. J. Stroke Cerebrovasc. Dis. 2003, 12, 232–236.
- Zonneveld, T.P.; Richard, E.; Vergouwen, M.D.I.; Nederkoorn, P.J.; de Haan, R.; Roos, Y.B.; Kruyt, N.D. Blood Pressure-Lowering Treatment for Preventing Recurrent Stroke, Major Vascular Events, and Dementia in Patients with a History of Stroke or Transient Ischaemic Attack. Cochrane Database Syst. Rev. 2018, 7, CD007858.
- Sps, T.; Group, S. Blood-Pressure Targets in Patients with Recent Lacunar Stroke: The SPS3 Randomised Trial. Lancet 2013, 382, 507–515.
- Petrone, L.; Nannoni, S.; Del Bene, A.; Palumbo, V.; Inzitari, D. Branch Atheromatous Disease: A Clinically Meaningful, yet Unproven Concept. Cerebrovasc. Dis. 2016, 41, 87–95.
- Caplan, L.R. Intracranial Branch Atheromatous Disease: A Neglected, Understudied, and Underused Concept. Neurology 1989, 39, 1246–1250.
- Amarenco, P.; Bogousslavsky, J.; Callahan, A.; Goldstein, L.B.; Hennerici, M.; Rudolph, A.E.; Sillesen, H.; Simunovic, L.; Szarek, M.; Welch, K.M.A.; et al. High-Dose Atorvastatin after Stroke or Transient Ischemic Attack. Curr. Atheroscler. Rep. 2007, 9, 96.
- Suzuki, T.; Natori, T.; Sasaki, M.; Miyazawa, H.; Narumi, S.; Ito, K.; Kamada, A.; Yoshida, M.; Tsuda, K.; Yoshioka, K.; et al. Evaluating Recanalization of Relevant Lenticulostriate Arteries in Acute Ischemic Stroke Using High-Resolution MRA at 7T. Int. J. Stroke 2021, 16, 1039–1046.
- Rudilosso, S.; Laredo, C.; Mancosu, M.; Moya-Planas, N.; Zhao, Y.; Chirife, O.; Chamorro, Á.; Urra, X. Cerebral Perfusion and Compensatory Blood Supply in Patients with Recent Small Subcortical Infarcts. J. Cereb. Blood Flow Metab. 2019, 39, 1326–1335.
- Rydén, L.; Sacuiu, S.; Wetterberg, H.; Najar, J.; Guo, X.; Kern, S.; Zettergren, A.; Shams, S.; Pereira, J.B.; Wahlund, L.-O.; et al. Atrial Fibrillation, Stroke, and Silent Cerebrovascular Disease: A Population-Based MRI Study. Neurology 2021, 97, e1608–e1619.
- Lee, J.H.; Kim, Y.J.; Moon, Y.; Cho, H.J.; Kim, H.Y. Acute Simultaneous Multiple Lacunar Infarcts: A Severe Disease Entity in Small Artery Disease. Eur. Neurol. 2012, 67, 303–311.
- Stewart, C.R.; Stringer, M.S.; Shi, Y.; Thrippleton, M.J.; Wardlaw, J.M. Associations Between White Matter Hyperintensity Burden, Cerebral Blood Flow and Transit Time in Small Vessel Disease: An Updated Meta-Analysis. Front. Neurol. 2021, 12, 647848.
- Wardlaw, J.; Bath, P.M.W.; Doubal, F.; Heye, A.; Sprigg, N.; Woodhouse, L.J.; Blair, G.; Appleton, J.; Cvoro, V.; England, T.; et al. Protocol: The Lacunar Intervention Trial 2 (LACI-2). A Trial of Two Repurposed Licenced Drugs to Prevent Progression of Cerebral Small Vessel Disease. Eur. Stroke J. 2020, 5.
- Roseborough, A.D.; Rasheed, B.; Jung, Y.; Nishimura, K.; Pinsky, W.; Langdon, K.D.; Hammond, R.; Pasternak, S.H.; Khan, A.R.; Whitehead, S.N. Microvessel Stenosis, Enlarged Perivascular Spaces, and Fibrinogen Deposition Are Associated with Ischemic Periventricular White Matter Hyperintensities. Brain Pathol. 2021, 32, e13017.
- Simpson, J.E.; Wharton, S.B.; Cooper, J.; Gelsthorpe, C.; Baxter, L.; Forster, G.; Shaw, P.J.; Savva, G.; Matthews, F.E.; Brayne, C.; et al. Alterations of the Blood-Brain Barrier in Cerebral White Matter Lesions in the Ageing Brain. Neurosci. Lett. 2010, 486, 246–251.
- Low, A.; Mak, E.; Rowe, J.B.; Markus, H.S.; O’Brien, J.T. Inflammation and Cerebral Small Vessel Disease: A Systematic Review. Ageing Res. Rev. 2019, 53, 100916.
- Wardlaw, J.M. Blood-Brain Barrier and Cerebral Small Vessel Disease. J. Neurol. Sci. 2010, 299, 66–71.
- Hainsworth, A.H.; Minett, T.; Andoh, J.; Forster, G.; Bhide, I.; Barrick, T.R.; Elderfield, K.; Jeevahan, J.; Markus, H.S.; Bridges, L.R. Neuropathology of White Matter Lesions, Blood-Brain Barrier Dysfunction, and Dementia. Stroke 2017, 48, 2799–2804.
- Wharton, S.B.; Simpson, J.E.; Brayne, C.; Ince, P.G. Age-Associated White Matter Lesions: The MRC Cognitive Function and Ageing Study. Brain Pathol. 2015, 25, 35–43.
- Wiseman, S.; Marlborough, F.; Doubal, F.; Webb, D.J.; Wardlaw, J. Blood Markers of Coagulation, Fibrinolysis, Endothelial Dysfunction and Inflammation in Lacunar Stroke versus Non-Lacunar Stroke and Non-Stroke: Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2014, 37, 64–75.
- Kelly, P.; Weimar, C.; Lemmens, R.; Murphy, S.; Purroy, F.; Arsovska, A.; Bornstein, N.M.; Czlonkowska, A.; Fischer, U.; Fonseca, A.C.; et al. Colchicine for Prevention of Vascular Inflammation in Non-CardioEmbolic Stroke (CONVINCE)—Study Protocol for a Randomised Controlled Trial. Eur. Stroke J. 2021, 6, 222–228.
- Chamorro, Á.; Amaro, S.; Castellanos, M.; Segura, T.; Arenillas, J.; Martí-Fábregas, J.; Gállego, J.; Krupinski, J.; Gomis, M.; Cánovas, D.; et al. Safety and Efficacy of Uric Acid in Patients with Acute Stroke (URICO-ICTUS): A Randomised, Double-Blind Phase 2b/3 Trial. Lancet Neurol. 2014, 13, 453–460.
- Ridker, P.M.; Thuren, T.; Zalewski, A.; Libby, P. Interleukin-1β Inhibition and the Prevention of Recurrent Cardiovascular Events: Rationale and Design of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 2011, 162, 597–605.
- Rudilosso, S.; Urra, X.; San Roman, L.; Laredo, C.; Lopez-Rueda, A.; Amaro, S.; Oleaga, L.; Chamorro, A. Perfusion Deficits and Mismatch in Patients with Acute Lacunar Infarcts Studied with Whole-Brain CT Perfusion. Am. J. Neuroradiol. 2015, 36, 1407–1412.
- Förster, A.; Kerl, H.U.; Wenz, H.; Brockmann, M.A.; Nölte, I.; Groden, C. Diffusion- and Perfusion-Weighted Imaging in Acute Lacunar Infarction: Is There a Mismatch? PLoS ONE 2013, 8, e77428.
- Förster, A.; Mürle, B.; Böhme, J.; Al-Zghloul, M.; Kerl, H.U.; Wenz, H.; Groden, C. Perfusion-Weighted Imaging and Dynamic 4D Angiograms for the Estimation of Collateral Blood Flow in Lacunar Infarction. J. Cereb. Blood Flow Metab. 2016, 36, 1744–1754.
- Huang, Y.C.; Lee, J.D.; Pan, Y.T.; Weng, H.H.; Yang, J.T.; Lin, L.C.; Tsai, Y.H. Perfusion Defects and Collateral Flow Patterns in Acute Small Subcortical Infarction: A 4D Dynamic MRI Study. Transl. Stroke Res. 2021.
- Smirnov, M.; Destrieux, C.; Maldonado, I.L. Cerebral White Matter Vasculature: Still Uncharted? Brain 2021, 144, 3561–3575.
- Jouvent, E.; Duering, M.; Chabriat, H. Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy: Lessons from Neuroimaging. Stroke 2020, 51, 21–28.
- Choi, J.C. Genetics of Cerebral Small Vessel Disease. J. Stroke 2015, 17, 7–16.
- Muiño, E.; Fernández-Cadenas, I.; Arboix, A. Contribution of “Omic” Studies to the Understanding of CADASIL. A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7357.
- Mancuso, M.; Arnold, M.; Bersano, A.; Burlina, A.; Chabriat, H.; Debette, S.; Enzinger, C.; Federico, A.; Filla, A.; Finsterer, J.; et al. Monogenic Cerebral Small-Vessel Diseases: Diagnosis and Therapy. Consensus Recommendations of the European Academy of Neurology. Eur. J. Neurol. 2020, 27, 909–927.
- Smith, E.E.; Biessels, G.J.; De Guio, F.; de Leeuw, F.E.; Duchesne, S.; Düring, M.; Frayne, R.; Ikram, M.A.; Jouvent, E.; MacIntosh, B.J.; et al. Harmonizing Brain Magnetic Resonance Imaging Methods for Vascular Contributions to Neurodegeneration. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 191–204.
- Lee, J.M.; Grabb, M.C.; Zipfel, G.J.; Choi, D.W. Brain Tissue Responses to Ischemia. J. Clin. Investig. 2000, 106, 723–731.
- Kim, J.G.; Choi, H.; Sohn, S.Y.; Kim, D.H.; Lee, S.J. Transient Ischemic Attacks Preceding Acute Lacunar Infarction. Eur. Neurol. 2016, 76, 278–283.
- Rudilosso, S.; Urra, X.; Chirife, O.; Chamorro, Á. Altered Brain Computed Tomography Perfusion in Patients with Fluctuating Lacunar Syndrome and Normal Magnetic Resonance Imaging. JAMA Neurol. 2016, 73, 348–349.
- Donnan, G.A.; O’Malley, H.M.; Quang, L.; Hurley, S.; Bladin, P.F. The Capsular Warning Syndrome: Pathogenesis and Clinical Features. Neurology 1993, 43, 957–962.
- Camps-Renom, P.; Delgado-Mederos, R.; Martínez-Domeño, A.; Prats-Sánchez, L.; Cortés-Vicente, E.; Simón-Talero, M.; Arboix, A.; Ois, Á.; Purroy, F.; Martí-Fàbregas, J. Clinical Characteristics and Outcome of the Capsular Warning Syndrome: A Multicenter Study. Int. J. Stroke 2015, 10, 571–575.
- Saposnik, G.; De Tilly, L.N.; Caplan, L.R. Pontine Warning Syndrome. Arch. Neurol. 2008, 65, 1375–1377.
- Muengtaweepongsa, S.; Singh, N.N.; Cruz-Flores, S. Pontine Warning Syndrome: Case Series and Review of Literature. J. Stroke Cerebrovasc. Dis. 2010, 19, 353–356.
- He, L.; Xu, R.; Wang, J.; Zhang, L.; Zhang, L.; Zhou, F.; Dong, W. Capsular Warning Syndrome: Clinical Analysis and Treatment. BMC Neurol. 2019, 19, 1–7.
- Rudilosso, S.; Olivera, M.; Esteller, D.; Laredo, C.; Amaro, S.; Llull, L.; Renú, A.; Obach, V.; Vera, V.; Rodríguez, A.; et al. Susceptibility Vessel Sign in Deep Perforating Arteries in Patients with Recent Small Subcortical Infarcts. J. Stroke Cerebrovasc. Dis. 2021, 30, 1–7.
- Jiang, S.; Yan, Y.; Yang, T.; Zhu, Q.; Wang, C.; Bai, X.; Hao, Z.; Zhang, S.; Yang, Q.; Fan, Z.; et al. Plaque Distribution Correlates with Morphology of Lenticulostriate Arteries in Single Subcortical Infarctions. Stroke 2020, 51, 2801–2809.
- Zhang, Z.; Fan, Z.; Kong, Q.; Xiao, J.; Wu, F.; An, J.; Yang, Q.; Li, D.; Zhuo, Y. Visualization of the Lenticulostriate Arteries at 3T Using Black-Blood T1-Weighted Intracranial Vessel Wall Imaging: Comparison with 7T TOF-MRA. Eur. Radiol. 2019, 29, 1452–1459.
- Wardlaw, J.M.; Doubal, F.; Armitage, P.; Chappell, F.; Carpenter, T.; Muñoz Maniega, S.; Farrall, A.; Sudlow, C.; Dennis, M.; Dhillon, B. Lacunar Stroke Is Associated with Diffuse Blood-Brain Barrier Dysfunction. Ann. Neurol. 2009, 65, 194–202.
- Blair, G.W.; Thrippleton, M.J.; Shi, Y.; Hamilton, I.; Stringer, M.; Chappell, F.; Dickie, D.A.; Andrews, P.; Marshall, I.; Doubal, F.N.; et al. Intracranial Hemodynamic Relationships in Patients with Cerebral Small Vessel Disease. Neurology 2020, 94, e2258–e2269.
- Stevenson, S.F.; Doubal, F.N.; Shuler, K.; Wardlaw, J.M. A Systematic Review of Dynamic Cerebral and Peripheral Endothelial Function in Lacunar Stroke versus Controls. Stroke 2010, 41, 434–442.
- Moreau, F.; Patel, S.; Lauzon, M.L.; McCreary, C.R.; Goyal, M.; Frayne, R.; Demchuk, A.M.; Coutts, S.B.; Smith, E.E. Cavitation after Acute Symptomatic Lacunar Stroke Depends on Time, Location, and MRI Sequence. Stroke 2012, 43, 1837–1842.
- Lee, K.J.; Jung, H.; Oh, Y.S.; Lim, E.Y.; Cho, A.H. The Fate of Acute Lacunar Lesions in Terms of Shape and Size. J. Stroke Cerebrovasc. Dis. 2017, 26, 1254–1257.
- Gattringer, T.; Valdes Hernandez, M.; Heye, A.; Armitage, P.A.; Makin, S.; Chappell, F.; Pinter, D.; Doubal, F.; Enzinger, C.; Fazekas, F.; et al. Predictors of Lesion Cavitation After Recent Small Subcortical Stroke. Transl. Stroke Res. 2020, 11, 402–411.
- Benjamin, P.; Trippier, S.; Lawrence, A.J.; Lambert, C.; Zeestraten, E.; Williams, O.A.; Patel, B.; Morris, R.G.; Barrick, T.R.; MacKinnon, A.D.; et al. Lacunar Infarcts, but Not Perivascular Spaces, Are Predictors of Cognitive Decline in Cerebral Small-Vessel Disease. Stroke 2018, 49, 586–593.
- del C. Valdés Hernández, M.; Maconick, L.C.; Muñoz Maniega, S.; Wang, X.; Wiseman, S.; Armitage, P.A.; Doubal, F.N.; Makin, S.; Sudlow, C.L.; Dennis, M.S.; et al. A Comparison of Location of Acute Symptomatic vs. “silent” Small Vessel Lesions. Int. J. Stroke 2015, 10, 1044–1050.
- Duering, M.; Csanadi, E.; Gesierich, B.; Jouvent, E.; Hervé, D.; Seiler, S.; Belaroussi, B.; Ropele, S.; Schmidt, R.; Chabriat, H.; et al. Incident Lacunes Preferentially Localize to the Edge of White Matter Hyperintensities: Insights into the Pathophysiology of Cerebral Small Vessel Disease. Brain 2013, 136, 2717–2726.
- Keith, J.; Gao, F.Q.; Noor, R.; Kiss, A.; Balasubramaniam, G.; Au, K.; Rogaeva, E.; Masellis, M.; Black, S.E. Collagenosis of the Deep Medullary Veins: An Underrecognized Pathologic Correlate of White Matter Hyperintensities and Periventricular Infarction? J. Neuropathol. Exp. Neurol. 2017, 76, 299–312.
More
