Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Chuan-Fa Chang and Version 2 by Bruce Ren.
An outbreak of SARS-CoV-2 coronavirus (COVID-19) first detected in Wuhan, China, has created a public health emergency all over the world. The pandemic has caused more than 340 million confirmed cases and 5.57 million deaths as of 23 January 2022. Although carbohydrates have been found to play a role in coronavirus binding and infection, the role of cell surface glycans in SARS-CoV-2 infection and pathogenesis is still not understood.
- blood group
- carbohydrate ligand
- COVID-19
- spike protein
1. Introduction
Since emerging from Wuhan, China in January 2020, the SARS-CoV-2 coronavirus has widely spread around the globe [1]. As of 23 January 2022, more than 210 countries have been affected by the pandemic, 340 million people have been diagnosed with the virus, and more than 5.57 million people have died. Although the number of new cases has been gradually decreasing, daily activities in most parts of the world have remained restricted. Therefore, deciphering the viral infection mechanism is of great urgency to facilitate the control and treatment of COVID-19.
The densely glycosylated spike (S) protein of SARS-CoV-2, a trimeric class I fusion protein with a metastable prefusion conformation [2][3][2,3], docks to enter host cells. When binding to a host-cell receptor, the S1 subunit triggers a dramatic structural rearrangement to fuse the viral membrane with the host-cell membrane, leading to receptor-dependent endocytosis [4][5][4,5]. These interactions destabilize the prefusion trimer and result in shedding of the S1 subunit and the transition of the S2 subunit into a stable postfusion conformation [6]. Several studies have shown the possible interactions between SARS-CoV-2 or the spike protein with the host receptors (angiotensin-converting enzyme 2 (ACE2) [7][8][9][10][7,8,9,10]; dipeptidyl peptidase 4 (DPP4) [11][12][11,12]; glucose-regulated protein 7 (GRP78) [13][14][13,14]); however, the discussion of cell surface glycan receptors used for SARS-CoV-2 viral binding and entry remained vague [15].
Cell surface glycan receptors are known to play a key role in mediating viral binding and infection. Jackson et al. indicated that the entry of foot-and-mouth disease virus (FMDV) into cells is initiated by the contact with heparin sulfate on the cell surface [16]. Sulfated polysaccharides extracted from sea algae have shown potential to prevent the infection of viruses, including herpes simplex virus (HSV), cytomegalovirus (CMV), human immunodeficiency virus (HIV), and enterovirus (EV) [17][18][19][20][21][17,18,19,20,21]. Lactoferrin, an 80 kDa iron-binding glycoprotein existing in several mucosal secretions [22][23][22,23], has been reported to inhibit the interaction of the capsid protein VP1 of EV71 with rhabdomyosarcoma cells [24][25][24,25]. In addition, sialic acids were reported as cell-surface ligands for many viral proteins of influenza virus, parainfluenza virus, reovirus type 3, adenovirus type 37, human rhinovirus 87, human enterovirus type 70 [26], EV-A71 [27], coxsackievirus A24 [28], and hepatitis A virus [29]. Human coronaviruses OC43, HKU1, and MERS were also shown to interact with sialic acids [30][31][32][33][30,31,32,33]. Clausen et al. indicated that the SARS-CoV-2 spike protein interacts with both cellular heparan sulfate and ACE2 through its receptor-binding domain [34]. Jayaprakash et al. also showed that the S1A domain of the SARS-CoV-2 spike protein may interact with sialosides by molecular modeling [35]. Furthermore, the N-glycans of DC-SIGN and L-SIGN that were identified to be the receptors for SARS-CoV-2 could influence the entry of coronavirus [36]. Therefore, it is important to study the role and function of cell surface glycans in the infection of SARS-CoV-2, which appears to be indispensable for understanding viral attachment, infection, and pathogenesis.
2. Carbohydrate Ligands for COVID-19 Spike Proteins
2.1. Sugar-Binding Profiling Analysis of SARS-CoV-2 Spike Proteins
We previously developed a homogeneous solution carbohydrate microarray in which polyacrylamide-based glycans are used to offer a multivalent environment to screen for specific carbohydrates. There are two advantages to this microarray. This platform can be carried out in a high-throughput manner because the washing step is not required during the screening [37] and is suitable to measure weak binding events that are typical in carbohydrate–protein interactions. So far, this platform has successfully demonstrated the carbohydrate-binding specificities of lectins, antibodies, influenza virus hemagglutinins, and influenza viral particles [38][39][38,39]. Using this solution carbohydrate microarray that contains 97 different glycans (Table 1), SARS-CoV-2 spike protein S1 subunits were bound specifically to 3-HSO3-Galβ (#16), GalNAcα1-3(Fucα1-2)Galβ (Blood Group A trisaccharide, #75), GlcNAcβ1-3(GlcNAcβ1-6)Galβ1-4Glcβ (#89), and Galα1-3(Fucα1-2)Galβ1-4GlcNAcβ (Blood Group B type 2 tetrasaccharide, #90) (Figure 1A). SARS-CoV-2 spike protein S2 subunits displayed preferential interactions with 3-HSO3-Galβ (#16), Galβ1-6Glcβ (melibiose, #44), Galβ1-3(Fucα1-4)GlcNAcβ (Lea, #58), Galβ1-3(GlcNAcβ1-6)GalNAcα (#74), and GlcNAcβ1-3(GlcNAcβ1-6)Galβ1-4Glcβ (#89) (Figure 1B). The carbohydrate binding preferences of spike protein S1 and S2 subunits with a relative intensity cutoff of 50% are listed in Table 2.
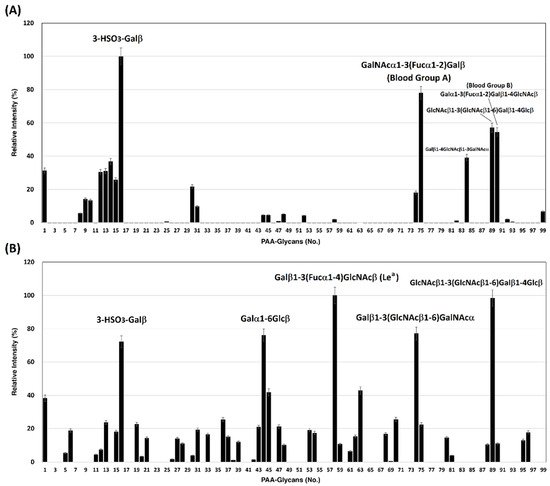

Figure 1. Binding profile of SARS-CoV-2 spike proteins with 97 biotin-PAA-sugars. (A) spike protein S1 subunit and (B) spike protein S2 subunit.
Table 1.
List of PAA-glycans.
| No. | Glycans | No. | Glycans | No. | Glycans |
|---|
Table 2.
The carbohydrate binding preferences of spike protein S1 and S2 subunits (cut off: relative intensity >50%).
| SARS-CoV-2 Spike Protein S1 Subunit | SARS-CoV-2 Spike Protein S2 Subunit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Glycan | No. | Glycan | |||||||
| 1 | PAA-biotin (backbone) | 34 | ||||||||
| 16 | 3-HSO3-Galβ | 16 | GlcNAcβ1-3Galβ | 67 | GlcNAcβ1-2Galβ1-3GalNAcα | |||||
| 3-HSO | 3 | -Galβ | 2 | β-GlcNAc | 35 | Galα1-4GlcNAcβ (αLacNAc) | 68 | NeuAcα2-3Galβ1-4GlcNAcβ | ||
| 75 | GalNAcα1-3(Fucα1-2)Galβ (Blood Group A trisaccharide) |
44 | Galα1-6Glcβ (melibiose) | 3 | α-Glucose | |||||
| 89 | GlcNAcβ1-3(GlcNAcβ1-6)Galβ1-4Glcβ | 36 | Glcα1-4Glcβ | 69 | NeuAcα2-3Galβ1-3Glcβn (3’Sialyl Lec) | |||||
| 58 | Galβ1-3(Fucα1-4)GlcNAcβ (Le | a | ) | 4 | β-Glucose | 37 | Galβ1-3GalNAcα, sp = -p-OC | |||
| 90 | Galα1-3(Fucα1-2)Galβ1-4GlcNAcβ (Blood Group B trisaccharide) | 6 | H4- | 70 | Galα1-3Galβ1-4GlcNAcβ, sp = -NHCOCH2NH- | |||||
| 74 | Galβ1-3(GlcNAcβ1-6)GalNAcα | 5 | α-Galactose | 38 | Galα1-2Galβ | 71 | GlcNAcα1-3Galβ1-3GalNAcα | |||
| 6 | β-Galactose | 39 | GlcNAcβ1-4GlcNAcβ | 72 | NeuAcα2-8NeuAcα2-8NeuAc, (NeuAcα2-8)3 | |||||
| 7 | α-Man-6-phosphate | 40 | GlcNAcβ1-4GlcNAcβ, sp = -NHCOCH2NH- | 73 | GlcNAcβ1-3Galβ1-3GalNAcα | |||||
| 8 | α-L-Rhamnose | 41 | NeuAcα2-6GalNAcα | 74 | Galβ1-3(GlcNAcβ1-6)GalNAcα | |||||
| 9 | β-GlcNAc | 42 | 3-HSO3-Galβ1-4GlcNAcβ | 75 | GalNAcα1-3(Fucα1-2)Galβ (Blood Group A), sp = (CH2)3NHCO(CH2)5NH- | |||||
| 10 | α-GalNAc | 43 | 3-HSO3-Galβ1-3GlcNAcβ | 76 | GlcNAcβ1-3Galβ1-4GlcNAcβ | |||||
| 11 | β-GalNAc | 44 | Galα1-6Glcβ (melibiose) | 77 | NeuAcα2-3Galβ1-3GalNAcα | |||||
| 12 | α-Fuc | 45 | NeuAcα2-8NeuAcα, (NeuAcα2-8)2 | 78 | GlcNAcβ1-3(GlcNAcβ1-6)GalNAcα | |||||
| 13 | α-NeuAc | 46 | Galβ1-2Galβ | 79 | Galα1-4Galβ1-4GlcNAcβ | |||||
| 14 | α-NeuAc-OCH2C6H4-p-NHCOOCH2 | 47 | 6-HSO3-Galβ1-4GlcNAcβ | 80 | GlcNAcβ1-4GlcNAcβ1-4GlcNAcβ, sp = -NHCOCH2NH- | |||||
| 15 | MurNAc-lactic acid-L-Ala-D-isoGln | 48 | NeuAcα2-3Gal | 81 | Galβ1-3(NeuAcα2-6)GalNAcα | |||||
| 16 | 3-HSO3-Galβ | 49 | 3-HSO3-Galβ1-3GalNAcβ (sulfate-TF) | 82 | Galβ1-3(NeuAcβ2-6)GalNAcα | |||||
| 17 | β-Mannose-PAA-biotin | 50 | GlcNAcβ1-3GalNAcα | 83 | NeuAcα2-3(NeuAcα2-6)GalNAcα | |||||
| 18 | α-NeuGc | 51 | GlcNAcβ1-6GalNAcα | 84 | Galβ1-4GlcNAcβ1-3GalNAcα | |||||
| 19 | 6-HSO3-GlcNAcβ | 52 | NeuGcα2-6GalNAcα | 85 | Fucα1-2Galβ1-3(Fucα1-4)GlcNAcβ (Leb) | |||||
| 20 | GalNAcα1-3Galβ | 53 | NeuAcβ2-6GalNAcα | 86 | Fucα1-2Galβ1-4(Fucα1-3)GlcNAcβ (Ley) | |||||
| 21 | Galα1-3Galβ | 54 | NeuAcα2-3GalNAcα | 87 | NeuAcα2-3Galβ1-3(Fucα1-4) GlcNAcβ (sialyl Lea) |
|||||
| 22 | Fucα1-2Galβ | 55 | GalNAcα1-3(Fucα1-2)Galβ (Blood Group A) | 88 | NeuAcα2-3Galβ1-4(Fucα1-3) GlcNAcβ (sialyl Lex) |
|||||
| 24 | Galβ1-4Glcβ (Lactose) | 57 | Fucα1-2Galβ1-4GlcNAcβ (H type2) | 90 | Galα1-3(Fucα1-2)Galβ1-4GlcNAcβ | |||||
| 25 | Galβ1-4GlcNAcβ (LacNAc) |
58 | Galβ1-3(Fucα1-4)GlcNAcβ (Lea) | 91 | Galβ1-3GlcNAcβ1-3Galβ1-4Glcβ | |||||
| 26 | Galβ1-3GalNAcα | 59 | Galβ1-4(Fucα1-3)GlcNAcβ (Lex) | 92 | (NeuAcα2-8)5-6 | |||||
| 27 | Fucα1-3GlcNAcβ | 60 | Fucα1-2Galβ1-3GlcNAcβ, Led (H type1) | 93 | Galβ1-4GlcNAcβ1-3(Galβ1- 4GlcNAcβ1-6)GalNAcα |
|||||
| 28 | Fucα1-4GlcNAcβ | 61 | NeuAcα2-3Galβ1-4Glcβ (3’Sialyl Lactose) | 94 | (NeuAcα2-6Galβ1-4GlcNAcβ1-2Man)2α1-3,6Manβ1-4GlcNAcβ1-4GlcNAcβ | |||||
| 33 | ||||||||||
| 89 | GlcNAcβ1-3(GlcNAcβ1-6)Galβ1-4Glcβ | 23 | Galβ1-3GlcNAc (Lec) | 56 | Galα1-3(Fucα1-2)Galβ (Blood Group B) | 89 | GlcNAcβ1-3(GlcNAcβ1-6)Galβ1-4Glcβ | |||
| 29 | GalNAcα1-3GalNAcβ | 62 | NeuAcα2-6Galβ1-4Glcβ (3’Sialyl Lactose) | 95 | GalNAcα-Ser | |||||
| 30 | GalNAcα1-3GalNAcα | 63 | 3-HSO3-Galβ1-4(Fucα1-3)GlcNAcβ (3’sulfate Lex) | 96 | GalNAcα1-3(Fucα1-2)Galβ1- 4GlcNAc |
|||||
| 31 | Galα1-3GalNAcα | 64 | 3-HSO3-Galβ1-3(Fucα1-4)GlcNAcβ (3’sulfate Lea) | 97 | Neu5Acα2-3(6-HSO3)Galβ1-4(Fucα1-3)GlcNAcβ (6Gal-HSO3-SiaLex) | |||||
| 32 | Galα1-3GalNAcβ | 65 | Galα1-4Galβ1-4Glcβ | 98 | Neu5Acα2-3Galβ1-4(Fucα1-3)(6-HSO3)GlcNAcβ (6GlcNAc-HSO3- SiaLex) |
Galβ1-3Galβ | 66 | Galα1-3Galβ1-4Glcβ | 99 | H2O |
2.2. SARS-CoV-2 Spike Proteins Interact with RBCs
The sugar-binding profiling analysis indicated that SARS-CoV-2 spike proteins displayed binding preference for blood-type antigens, including Group A (#75 in Figure 1A), blood Group B (#90 in Figure 1A), and Lea (#58 in Figure 1B). To investigate if the binding preference of SARS-CoV-2 spike proteins correlated with the viral infection or pathogenesis, we examined RBCs that are known to express different blood groups, including group A (Lea+/Leb−), B (Lea+/Leb−), and O (Lea−/Leb+). The binding assay was conducted using fluorescence-activated cell sorting (FACS). The results indicated that the SARS-CoV-2 spike protein S1 subunit binds strongly to group A RBCs, moderately to group B RBCs, and relatively weakly to group O RBCs (Figure 2). The SARS-CoV-2 spike protein S2 subunit displayed higher binding signals with Lea+ RBCs than with Lea− RBCs (Figure 2). This observation was consistent with the analysis of carbohydrate microarray, which showed that the spike protein S1 subunit shows a higher preference for blood group A and B RBCs. The binding preference is related to the glycan structures existing on the surface of RBCs.
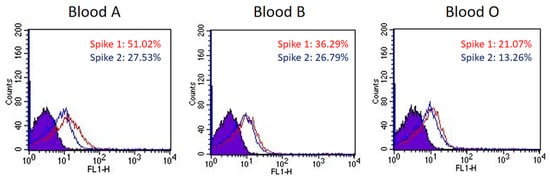

Figure 2. The binding preference of SARS-CoV-2 spike proteins to blood group A, B, and O RBCs. The histograms of spike protein S1 subunits binding with blood group A, B, and O RBCs show a 51.02%, 36.29%, and 21.07% shift in mean fluorescence intensity (MFI), respectively (red line). The histograms of spike protein S2 subunits binding with blood group A, B, and O RBCs show a 27.53%, 26.79%, and 13.26% shift in MFI, respectively (blue line).
2.3. Carbohydrate Derivatives Interfere with Interaction of SARS-CoV-2 Spike Protein S1 Subunit and Blood Group A RBCs
Carbohydrate analogs are able to interrupt the interaction between microorganisms and host cells by associating with glycoproteins on the surface of either host cells or microorganisms. For example, heparin sulfate mimetics exhibit antiviral activity against dengue virus by inhibiting the virus adsorption on host cells to prevent virus entry [40]. Neuraminidase inhibitors are used for anti-influenza therapy by inhibiting the neuraminidase activity to modify the cell surface glycans, which results in prevention of virions spreading to neighboring cells [41]. Since SARS-CoV-2 spike proteins show a binding preference to blood groups A and B, it is important to examine whether carbohydrate analogs interfere with the interaction between spike proteins and RBCs, especially galactin-3 inhibitors [42]. Lactose and three other carbohydrate derivatives were examined, including compounds 1 and 2 and TD-139. Each of them was preincubated with SARS-CoV-2 spike protein S1 subunit, followed by the addition of blood group A RBCs to the assay mixture (Figure 3A–D). The FACS analysis indicated that compound 1 significantly prevented the binding of spike protein S1 subunit with RBCs up to 45% (Figure 3E, p < 0.01). However, lactose and compound 2 enhanced the interaction of spike protein S1 subunit to RBCs by 53% and 26%, respectively (Figure 3E). Interestingly, TD139, a potent inhibitor of galactin-3, exhibited no effect on the spike protein S1 subunit–RBC interaction. The binding inhibition results indicated that carbohydrate analogs containing both sulfate and LacNAc groups reduce the binding affinity between the SARS-CoV-2 spike protein S1 subunit and host cells.
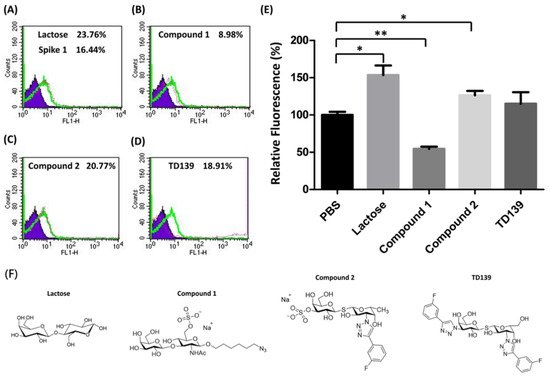

Figure 3. The binding efficiency of the SARS-CoV-2 spike protein S1 subunit to blood group A RBCs affected by carbohydrate derivatives. (A) The histogram of spike protein S1 subunit binding with blood group A RBC cells shows a 16.44% shift in MFI without carbohydrate inhibitors. Lactose and three sulfated carbohydrate derivatives were preincubated with spike protein S1 subunit then subjected to the binding assay. The histograms of spike protein S1 subunit binding with blood group A RBC cells are shown (green line). The MFI shifts of blood group A RBC cells with carbohydrate derivatives tested are (A) lactose, 23.76%; (B) compound 1, 8.98%; (C) compound 2, 20.77%; and (D) TD139, 18.91%. (E) The relative fluorescence shows the binding efficiency of the spike protein S1 subunit to blood group A RBCs influenced by carbohydrate derivatives. (F) Structures of lactose and three sulfated carbohydrate derivatives. * indicated p < 0.05; ** indicated p < 0.01.
2.4. Blood Group A Antigen on ACE2
Since ACE2 is widely recognized as the major binding target for SARS-CoV-2 spike proteins, it is worth investigating whether the host receptor ACE2 contains the blood group A antigen. ACE2 protein was obtained from the extraction of lung tissues of a blood group A person. The ACE2 protein was immunoprecipitated with anti-ACE2 antibody. Western blotting analysis indicated that the glycoprotein ACE2 in the lung tissue of the blood group A person contained the carbohydrate chains of the blood group A antigen (Figure 4A).
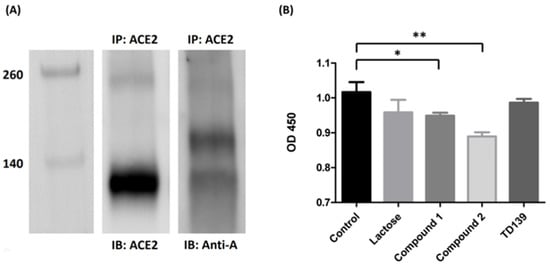

Figure 4. ACE2 contains blood group A antigen. (A) Western blotting analysis of ACE2 from the lung tissue of a blood group A patient. Lane 1: markers. Lane 2: the Western blotting of the lung tissue from blood group A patient immunoprecipitated with anti-ACE2 antibody and immunoblotted with anti-ACE2 antibody. Lane 3: the Western blotting of the lung tissue from blood group A patient immunoprecipitated with anti-ACE2 antibody and immunoblotted with anti-A antibody. (B) The binding inhibition assay of lactose or sulfated carbohydrate derivatives in blocking of the SARS-CoV-2 spike protein S1 subunit to ACE2. Compounds 1 and 2 showed a reduction in binding efficiency of ACE2 with the spike protein S1 subunit by 6.7% and 12.5%, respectively. TD139 exhibited no effects in spike protein S1 subunit–ACE2 interaction. * indicated p < 0.05; ** indicated p < 0.01.
2.5. Carbohydrate Derivatives Interfere with Interaction of SARS-CoV-2 Spike Protein S1 Subunit and ACE2
To study if it is possible to disrupt the interaction between SARS-CoV-2 spike protein and ACE2, an ELISA assay was performed to determine the binding inhibition efficiency of carbohydrate derivatives. SARS-CoV-2 spike protein S1 subunits were initially coated on 96-well microplates and incubated with carbohydrate derivatives. After washing away the nonbinding carbohydrate derivatives, human ACE2 proteins prepared from lung tissue lysate by immunoprecipitation were added. The quantities of ACE2 bound on spike protein S1 subunits were determined by ELISA assay. Both compounds 1 and 2 showed a significant decrease in the binding efficiency of ACE2 with the spike protein S1 subunit by 6.7% and 12.5%, respectively (Figure 4B). However, TD139 exhibited no effect on the interaction between spike protein S1 subunit and ACE2. Our results suggested that the specific carbohydrate modifications on ACE2 might be responsible for its binding to SARS-CoV-2 spike protein S1 subunit. Further studies are in progress to decipher the inhibitive effects of these carbohydrate derivatives on the interaction of spike protein S1 subunit and ACE2.
