In mice and rats, the intronless gene
Dux, encoded by a 4.9 kb retrogene array of more than 28 copies, is located at chromosome 10, whereas the 23
Dux paralog copies in human double homeobox 4 (
DUX4); only 1–12 bases different from mouse
Dux have been identified, in which 14 encoding mRNAs are transcribed in 2-cell-stage embryos as
Dux [20]. The further study of these 14 mRNAs’ expression regulation will help us to understand the difference between
Dux and its paralog. The intronless orthologs to human
DUX4 are also found in other primates, elephants, hyraxes, and tenrecs. Similar to
Dux, the
DUX4 family also contains a 3.3 kb copies array (D4Z4) located at the distal end of chromosome 4
[21]. An intron-containing variant, double homeobox B-like (
Duxbl), is only found in rodents, and its pseudogene is found in primates
[21]. The intronless
Dux/
DUX4 was hypothesized to arise from the common intron-containing ancestor in placental mammals through the reverse-transcription and retrotransposition models
[22]. During convergent evolution, environment pressure may further dictate the location of gene insertion. Genomic microsatellite organizations of
Dux and
DUX4 are usually located at the heterochromatinized regions, and their expression is often silenced in most types of cells, including embryonic stem cells
[9][23][9,23]. Epigenetic regulations, such as DNA methylation, are a key determinant for their expression
[23]. Despite the considerable sequence divergence in their DNA-binding domains, DUX and DUX4 shared a more conserved homeodomain 2 (HD2) domain to recognize the 5′-TGA-3′ motif
[24] and activate a subset of genes associated with cleavage-stage embryos
[9]. Interestingly, the swapping of the homeodomain 1 (HD1) and HD2 regions of DUX4 with the corresponding regions from DUX substantially attenuates the activation of Zscan4c and MERVL induced by DUX expression in mouse muscle cells
[25]. Additionally, mouse Dux is myotoxic and shares a partial functional homology with its human paralog DUX4, the aberrant expression of which is linked to facioscapulohumeral muscular dystrophy
[26], which further confirms the conserved roles of Dux/DUX4 in gene regulation.
3. The Regulation of Dux Expression
Dux mRNAs are expressed at the early 2-cell stage in mice during the minor zygotic genome activation (ZGA) stage, whereas it activates several genes during major ZGA, similar to DUX’s homolog DUX4
[20]. The overexpression of Dux in mESCs resulted in changes in gene expression and endowed these cells with totipotency
[9][25][27][9,25,27]. Given its critical role in ZGA and the ESCs to 2CLCs transition, Dux expression must be tightly regulated to ensure the correct developmental progression of cells and tissues. Although transcriptome studies on mouse cells expressing Dux have been widely carried out in vivo and in vitro, the mechanisms for the regulation of
Dux gene expression remain elusive. Thus,
rwe
searchers will discuss the recent studies revealing the mechanisms modulating Dux expression, involving transcriptional regulation by transcription factors, epigenetic modifications, signaling pathways and 3D genome conformation.
3.1. Transcriptional Regulation of Dux Expression by Transcription Factors
3.1.1. DPPA2/4
Given that Dux is only expressed in the first or minor wave of ZGA, there must be other factors involved in the precise activation of its expression. By screening epigenetic factors that can increase 2CLCs population in normal ESCs, developmental pluripotency associated 2 (DPPA2) and developmental pluripotency associated 4 (DPPA4) have been identified as upstream factors of Dux that initiate 2C-like transcription
[28]. DPPA2/4 are small putative DNA-binding proteins expressed exclusively in preimplantation embryos and pluripotent cells
[29]. The overexpression of DPPA2/4 can activate an early 2-cell transcriptome, a similar pattern to that seen in mESCs. Additionally, ChIP-seq data reveal that DPPA2/4 can directly bind to the Dux repeats and the promoter region. However, DPPA2 or DPPA4 cannot work alone to activate Dux expression; these two factors must be present in equimolar amounts to transactivate Dux
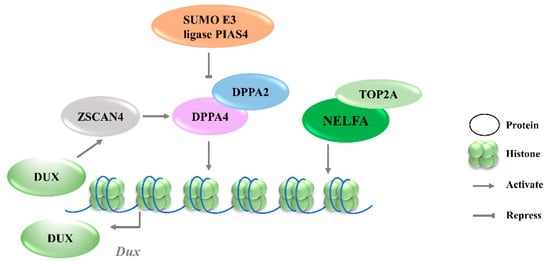
[30]. Recent studies further revealed that the DPPA2 activity is negatively regulated by a small ubiquitin-like modifier (SUMO) E3 ligasePIAS4, through the SUMOylation of DPPA2, which leads to its degradation (
Figure 1). Either PIAS4 knockout or DPPA2/4 overexpression is sufficient to activate a 2C-like transcriptional program; the expressions of
MERVL and other classic 2C-specific genes, including
Dux, N-acetyltransferase 8 family member 2 (
Cml2),
Zfp352, and zinc finger and SCAN domain containing 4D (
Zscan4d), are then upregulated
[31].
Figure 1. Transcriptional regulation of Dux expression by transcription factors. DPPA2/4 and NELFA transcriptionally activate Dux. Meanwhile, DUX can activate Zscan4 which will upregulate DPPA2/4. PIAS4 will repress DPPA2/4 expression through the SUMOylating of DPPA2. DPPA2/4, developmental pluripotency associated 2/4; PIAS4, protein inhibitor of activated STAT 4; NELFA, negative elongation factor complex member A; ZSCAN4, SCAN domain containing 4; TOP2A, DNA topoisomerase 2a; DUX, double homeobox.
3.1.2. NELFA
Negative elongation factor complex member A (NELFA) was another transcription factor reported to drive the progression to the 2CLCs state by activating Dux
[32]. NELFA is a member of the NELF complex family that regulates RNA polymerase II pausing
[33]. Unlike the enrichment of DPPA2/4 at the Dux locus, ChIP-seq data showed the low enrichment of NELFA at this locus
[34]. However, upon NELFA induction, NELFA located at the Dux locus was responsible for the chromatin opening and the transcriptional activation of Dux. Specifically, the interaction of NELFA with DNA topoisomerase 2a (Top2a) is essential for NELFA to activate Dux; Dux will be silenced in Top2a-deficient cells even when NELFA is overexpressed (
Figure 1)
[32]. The role of NELFA remains controversial, as other studies indicate that NELFA is a direct target of DUX rather than a driver of Dux
[34]. More data from Nelfa knock-out mESCs or embryos will help to clarify the role of NELFA in Dux regulation.
3.1.3. ZSCAN4C
Zinc finger and SCAN domain containing protein 4 C (ZSCAN4C), which shares a similar expression pattern to MERVL in normal mESCs, has also been identified as a 2CLCs marker
[35]. The activation of MERVL by ZSCAN4C is associated with promoting enhancer activity and enhancing histone modification deposition related to gene activation at MERVL LTR loci
[36]. Although Dux activation was observed after the overexpression of Zscan4c, the ChIP-seq data do not show the direct binding of ZSCAN4C in the Dux promoter region, suggesting an indirect transcriptional activation of Dux by ZSCAN4C binding
[36]. However, ZSCAN4 has been demonstrated to facilitate gene expression by inducing global DNA demethylation through silencing the DNA methylation ubiquitin-like components, UHRF1and DNMT1, indicating an additional regulatory layer of Dux by ZSCAN4 (
Figure 2A)
[37]. Moreover, Dux regulators, including DPPA2 and DPPA4, are upregulated by ZSCAN4 overexpression, reflecting that ZSCAN4, DPPA2/4, and DUX may reinforce each other’s expressions and form a positive feedback loop to strengthen 2-cell-like state transition (
Figure 1).
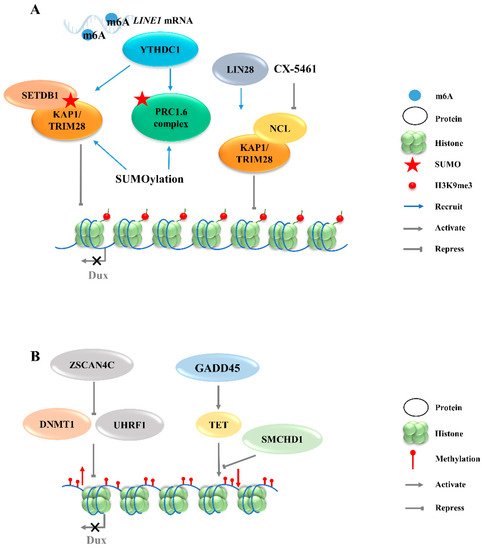
Figure 2. Regulation of Dux expression by epigenetic modifications. (A): Dux expression regulated by histone modifications transcription. The activation of H3K9me3 regulators caused by YTHDC1 and LIN28 will enhance the H3K9me3 levels and silence Dux expression finally. (B): Dux expression regulated by DNA modifications. DNA methylations caused by DNMT1/UHRF1 and TET repress Dux expression. LINE1, long interspersed nuclear element-1; SETDB1, SET domain bifurcated histone lysine methyltransferase 1; KAP1, KRAP-associated transcriptional repressor; TRIM28, tripartite motif-containing protein 28; PRC1.6, polycomb-repressive complexes 1.6; NCL, nucleolin; ZSCAN4C, SCAN domain containing 4C; DNMT1, DNA methyltransferase 1; UHRF1, ubiquitin like with PHD and ring finger domains 1; GADD45, growth arrest and DNA damage 45; TET, ten-eleven translocation; SMCHD1, structural maintenance of chromosomes flexible hinge domain containing 1.
3.2. Regulation of Dux Expression by Epigenetic Modifications
3.2.1. H3K9 Methylation
The totipotent 2CLCs have also been reported to exhibit increased histone modifications in H3K27ac, H3K4me1, and H3K4me3, as compared with ESCs [38]. Although these histone modifications are associated with transcriptional activation, no evidence exists to show that Dux expression will be directly regulated by these modifications. The downregulations of chromatin modifiers such as LSD1 and chromatin assembly factor 1 (CAF-1) facilitate MERVL activation [15][39]. Furthermore, MERVL requires lysine (K)-specific demethylase 1A (KDM1A, also as LSD1)—a histone lysine-specific demethylase, a KRAP-associated transcriptional repressor (KAP1), and G9A—a H3K9 histone methyltransferase—for epigenetic repression in normal mESCs [27][40][41]. Likewise, there is no direct evidence showing that the expression of Dux can be regulated by these chromatin modifiers. Recently, LIN28, an RNA-binding protein, was identified as able to repress Dux by an epigenetic program (The totipotent 2CLCs have also been reported to exhibit increased histone modifications in H3K27ac, H3K4me1, and H3K4me3, as compared with ESCs [38].Although these histone modifications are associated with transcriptional activation, no evidence exists to show that Dux expression will be directly regulated by these modifications. The downregulations of chromatin modifiers such as LSD1 and chromatin assembly factor 1 (CAF-1) facilitate MERVL activation [15,39]. Furthermore, MERVL requires lysine (K)-specific demethylase 1A (KDM1A, also as LSD1)—a histone lysine-specific demethylase, a KRAP-associated transcriptional repressor (KAP1), and G9A—a H3K9 histone methyltransferase—for epigenetic repression in normal mESCs [27,40,41]. Likewise, there is no direct evidence showing that the expression of Dux can be regulated by these chromatin modifiers. Recently, LIN28, an RNA-binding protein, was identified as able to repress Dux by an epigenetic program (Figure 2A). H3K9me3 levels were decreased at Dux and its downstream targets, and thus de-repressed Dux expression in Lin28 knockout cells [11]. However, the mechanisms underlying how Lin28 regulates H3K9me3 remain elusive. It is worth noting that Lin28a depletion releases Dux repression by reducing the occupancy of Nucleolin/tripartite motif-containing protein 28 (NCL/TRIM28) in the Dux region. TRIM28 is also known as KAP1, which was demonstrated to repress Dux expression in a long interspersed nuclear element-1 (LINE1)-dependent manner in mESCs [27][42]. LINE1 are Class I transposable elements, which can repress Dux expression by interacting with NCL and KAP1 in mESCs [42]. Mechanistically, after LINE1 RNA is methylated by METTLE3, the m6A-modified LINE1 RNA then works as a scaffold recognized by the YTH domain containing 1 (YTHDC1), which further recruits H3K9me3 regulators, including SET domain bifurcated histone lysine methyltransferase 1 (SETDB1) and KAP1, to the locus of Dux, inhibiting its expression [43][44]. Furthermore, polycomb-repressive complexes (PRCs) bind LINE1 RNA and act as an essential partner for A). H3K9me3 levels were decreased at Dux and its downstream targets, and thus de-repressed Dux expression in Lin28 knockout cells [11]. However, the mechanisms underlying how Lin28 regulates H3K9me3 remain elusive. It is worth noting that Lin28a depletion releases Dux repression by reducing the occupancy of Nucleolin/tripartite motif-containing protein 28 (NCL/TRIM28) in the Dux region. TRIM28 is also known as KAP1, which was demonstrated to repress Dux expression in a long interspersed nuclear element-1 (LINE1)-dependent manner in mESCs [27,42]. LINE1 are Class I transposable elements, which can repress Dux expression by interacting with NCL and KAP1 in mESCs [42]. Mechanistically, after LINE1 RNA is methylated by METTLE3, the m6A-modified LINE1 RNA then works as a scaffold recognized by the YTH domain containing 1 (YTHDC1), which further recruits H3K9me3 regulators, including SET domain bifurcated histone lysine methyltransferase 1 (SETDB1) and KAP1, to the locus of Dux, inhibiting its expression [43,44].Furthermore, polycomb-repressive complexes (PRCs) bind LINE1 RNA and act as an essential partner for Dux gene repression [45]. SUMO modification enhances the H3K9me3 levels on a genome-wide scale, including the Dux locus, and facilitates the recruitment of PRC1.6 and KAP/SETDB1 complexes to the locus to repress gene repression [ 45 ].SUMO modification enhances the H3K9me3 levels on a genome-wide scale, including the Dux locus, and facilitates the recruitment of PRC1.6 and KAP/SETDB1 complexes to the locus to repress Dux gene expression ( gene expression (Figure 2A) [46]. In fact, Ythdc1 depletion results in a global decrease in the SETDB1-mediated H3K9me3 enrichment, which is accompanied by the re-activation of MERVL and Dux [43]. However, Ythdc1-depleted cells still retain the ability to re-activate many retrotransposons upon Dux removal, indicating a parallel regulation pattern between Ythdc1 and Dux with regard to retrotransposon regulation [44]. Due to the sequence differences in LINE1 among species, it is not clear whether LINE1 has similar effects in other mammals, including A) [46]. In fact, Ythdc1 depletion results in a global decrease in the SETDB1-mediated H3K9me3 enrichment, which is accompanied by the re-activation of MERVL and Dux [43]. However, Ythdc1-depleted cells still retain the ability to re-activate many retrotransposons upon Dux removal, indicating a parallel regulation pattern between Ythdc1 and Dux with regard to retrotransposon regulation [44]. Due to the sequence differences in LINE1 among species, it is not clear whether LINE1 has similar effects in other mammals, including Homo sapiens [47]. [ 47 ]。
3.2.2. Histone Variants
The chromatin assembly factor CAF-1 has been reported to repress MERVL [39], and recent studies revealed its role in establishing the modification of the non-canonical histone variant H3.3, which has been reported to co-enrich with H3K9me3 to silence ERVs in mESCs [48]. The knockout of p150, a subunit of the CAF-1 complex, leads to a decrease in the total H3.3 enrichment, accompanied by the upregulation of Dux and MERVL. ChIP-seq data further confirm that H3.3 is enriched at the The chromatin assembly factor CAF-1 has been reported to repress MERVL [39], and recent studies revealed its role in establishing the modification of the non-canonical histone variant H3.3, which has been reported to co-enrich with H3K9me3 to silence ERVs in mESCs [48].The knockout of p150, a subunit of the CAF-1 complex, leads to a decrease in the total H3.3 enrichment, accompanied by the upregulation of Dux and MERVL. ChIP-seq data further confirm that H3.3 is enriched at the Dux locus and represses Dux expression [49]. However, the incorporation of H3.3 chaperones HIRA, ATRX or DAXXis not necessary per se for the function of H3.3 in Dux repression, indicating the existence of other H3.3 chaperones that may regulate Dux expression [49]. locus and represses Dux expression [49]. However, the incorporation of H3.3 chaperones HIRA, ATRX or DAXXis not necessary per se for the function of H3.3 in Dux repression, indicating the existence of other H3.3 chaperones that may regulate Dux expression [49].
3.2.3. DNA Methylation
In addition to histone modification, there are additional epigenetic mechanisms that regulate the activity of Dux. Typically occurring at the cytosine in CpG, 5-Methylcytosine (5mC) is a critical modification in the development and differentiation of cell lineages by blocking gene transcription [50]. The structural maintenance of chromosomes flexible hinge domain containing 1 (SMCHD1) cooperates with ten-eleven translocation (TET) proteins to negatively regulate the activities in DNA demethylation (Figure 2B). The removal of SMCHD1 from mESCs induces TET-dependent demethylation, preferentially at SMCHD1 targeting sites, along with the activation of B). The removal of SMCHD1 from mESCs induces TET-dependent demethylation, preferentially at SMCHD1 targeting sites, along with the activation of Dux and the and the Dux pseudogene ( pseudogene (Gm4981) [12][51]. The siRNA-mediated knockdown of Smchd1 in zygotes leads to a continued overexpression of Dux through the 8-cell stage. In addition, the presence of an unmethylated state of the ) [12,51]. The siRNA-mediated knockdown of Smchd1 in zygotes leads to a continued overexpression of Dux through the 8-cell stage. In addition, the presence of an unmethylated state of the Dux promoter region in the 2-cell stage indicates that the initial activation of Dux DNA is demethylation-dependent [51][52]. However, the mechanism of re-methylation of the promoter region in the 2-cell stage indicates that the initial activation of Dux DNA is demethylation-dependent [51,52]. However, the mechanism of re-methylation of the Dux locus occurring in the later stages of development remains elusive. Further studies are also needed to address whether SMCHD1 can inhibit TET proteins to modulate the DNA demethylation process. Growth arrest and DNA damage 45 (GADD45) is another regulator of TET-mediated DNA demethylation. The triple-knockout (TKO) of Gadd45a, b, and g in ESCs causes locus-specific DNA hypermethylation, along with the downregulation of 2C-specific genes, including Dux. The transient overexpression of Gadd45a, Gadd45b, or Gadd45g, individually or together, in a TKO background recovered Dux expression back to the control level [53]. These findings suggest that TET enzymes may play a dual role in regulating Dux expression when engaging with different partners. On the one hand, TET enzymes may play a repressive role when SMCHD1 represses the TET de-methylation function to silence Dux expression [12]. On the other hand, TET enzymes can play a promoting role in activating Dux expression through demethylation via physical interaction with GADD45 (Figure 2B).
3.3. Signaling Pathways Involved in Regulation of Dux Expression
3.3.1. Retinoic Acid Pathway
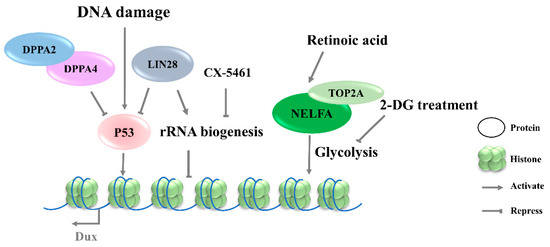
Retinoic acid (RA), a derived form of vitamin A (VitA), is involved in a variety of biological functions, including embryogenesis [54] and cell differentiation [55]. RA has been reported to initiate the reprogramming of ESCs to 2CLCs by co-activating Dux and Duxbl1, but the mechanism underlying Dux regulation by RA is not clear [56][57]. Iturbide et al. demonstrated that low concentrations of RA are sufficient to induce 2CLCs in cooperation with RARγ [58]. Interestingly, RA also participates in the NELFA-mediated 2C-like state of mESCs (Figure 3), but the downstream receptors have not been identified [56].
Figure 3. Signaling pathways involved in the regulation of Dux expression. DNA damage lead to P53 activation and then upregulates Dux expression. CX-5461 can decrease rRNA biogenesis and activate Dux expression. Retinoic acid participates in the NELFA-mediated Dux activation, and 2-DG inhibited glycolytic flux and activated Dux expression. DPPA2/4, developmental pluripotency associated 2/4; NELFA, negative elongation factor complex member a; TOP2A, DNA topoisomerase 2a; P53, tumor protein 53.
3.3.2. Glycolysis Pathway
The 2CLCs exhibit decreased glycolytic and respiratory activity and lower levels of reactive oxygen species, but with an increased glucose uptake, suggesting a distinct metabolic state arising during the transition from ESCs to 2CLCs [59][60]. Regarding the mechanism underlying the NELFA control of Dux activation, the glycolysis pathway was found to be downregulated in NELFA-induced 2CLCs, along with a decreased chromatin accessibility of glycolysis-associated genes (Figure 3). Further investigation confirmed the inhibition of glycolytic flux by 2-deoxy-d-glucose (2-DG), which promoted the 2CLC state transition; naïve pluripotency stabilizer PR/SET domain 14 (Prdm14) serves as a barrier in this transition. The upregulation of 2C genes has been observed upon Prdm14 knockdown in mESCs [32][61]. However, it is unclear whether the alteration of the energy metabolism pathway is the driver of Dux activation, or if it is a consequence of Dux expression.
3.3.3. DNA-Damage Response Pathway
Although DNA-damage-induced cellular differentiation was investigated in detail in ESCs [34][62], its role in totipotency regulation remains incompletely understood. Recent studies have shown that Dux activation in mESCs is initiated by the DNA-damage response (DDR) pathway, in which DNA damage-induced p53 activation plays an important role in regulating Dux expression (Figure 3). Double-strand DNA breaking induced by doxorubicin, PpoI endonuclease, hydroxyurea–aphidicolin [34], or UV [63] in mESCs results in higher levels of Dux activation and an increased MERVL-positive cell population. Consistent with these findings, p53 deficiency led to an almost complete loss of MERVL/Zscan4+ mESCs [63], accompanied by Dux repression [34]. ChIP-seq data on p53 further showed the direct binding of P53 in the Dux locus. In addition, Lin28 or Dppa2/4 deletion-mediated Dux activation is associated with p53 activation [11][28]. In contrast, in vivo data reveal that p53-knockout embryos are still positive for anti-DUX antibody staining, indicating that Dux activation is more complicated in in vivo settings [28]. The P53 regulation of mouse Dux and human DUX4 is conserved; however, the underlying molecular mechanisms are quite different. Although the intronless mouse Dux does not contain primate-specific LTR10C, which can be targeted by p53, p53 can bind to the Dux promoter directly to activate the transcription of mouse Dux [34].
3.3.4. rRNA Biogenesis Pathway
Protein synthesis heterogeneity requires the ribosome to be assembled in a cell-type-specific manner. Thus, differences in ribosome biogenesis can cause specialized mRNA subsets to be translated, which indirectly determine cell identity. Recent studies indicate that the inhibition of rRNA biogenesis can promote the state transition from ESCs to 2CLCs [11][13]. CX-5461, an RNA Pol I inhibitor, can decrease rRNA biogenesis and disrupt the nucleolar integrity and formation of peri-nucleolar heterochromatin (PNH). Upon the disruption of nucleolar integrity, the loss of NCL/TRIM28 complex from PNH causes changes in epigenetic modification, leading to the release of Dux (Figure 4) [13]. Transcription factor LIN28 has been reported to bind to RNAs in the nucleolus, and its loss impeded ribosome assembly and recapitulated CX-5461-induced 2CLCs molecular phenotypes [11]. In addition, rRNA repression will activate p53, an effector of DNA-damage response pathway [34]. Nevertheless, the connection to the mechanism of Dux production by transcriptional regulation and specific pathways observed in Dux activation remains to be elucidated. Meanwhile, how the different signaling pathways cooperate during Dux activation requires further elucidation.
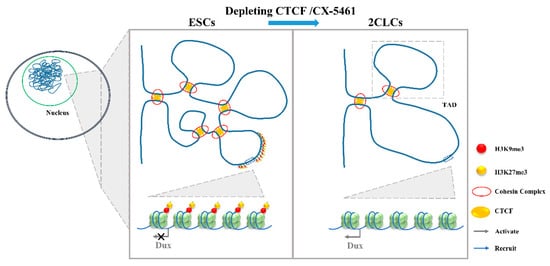
Figure 4. The 3D genome conformation determines the expression of Dux. The disruption of chromatin organization by the depletion of CTCF or addition with CX-5461 will lead to the lower TAD strength and TAD insulation and, finally, upregulate Dux expression. ESCs, embryonic stem cells; 2CLCs, 2-cell-like cells; CTCF, CCCTC-binding factor; TAD, topologically associating domains.
3.4. 3D Genome Conformation
Gene expression regulation is relevant to chromatin structure [64], and Hi-C studies profiling the 3D chromatin structure of the genome could describe the evolutionarily conserved topologically associating domains (TADs) that were correlated with gene expression. Combined with mouse and human models, TADs have been linked to gene regulation. During the mESC to 2CLC transition, a lower TAD strength and TAD insulation have been observed [39]. Consistent with this is the disruption of chromatin organization by the depletion of CCCTC-binding factor (CTCF)/Cohesin or by culturing with CX-5461 upregulate Dux expression and promote a 2-cell-like program [13][14]. It is worth noting that the attenuation of H3K9me3 and H3K27me3 in CX-5461-treated cells is associated with the reinforcement of a repressed chromatin state [65]. The expression of human DUX4, the C-terminal region, which shares a high similarity with mouse DUX, can recruit CBP and P300 to induce local chromatin relaxation, accompanied with a global increase in H3K27 acetylation [66]. These results indicate that epigenetic modification is important for the chromatin state change [67]. Chromatin relaxation facilitates the binding of transcription factors to the Dux locus, which might be the first step required to promote Dux expression [14][39]. The expression of DUX increases the active histone modifications and chromatin accessibility [9], and this positive feedback loop might be required to promote the 2C-like state (Figure 4).
locus occurring in the later stages of development remains elusive. Further studies are also needed to address whether SMCHD1 can inhibit TET proteins to modulate the DNA demethylation process. Growth arrest and DNA damage 45 (GADD45) is another regulator of TET-mediated DNA demethylation.