Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Bruce Ren and Version 1 by Silvina Odete Bustos.
Tumor microenvironment (TME) is a complex of many cell types and extracellular matrix that play an active role in regulating and sustaining melanoma tumor progression. In this context, tThe secretion of several molecules, by secretory autophagy or exosome release, stimulates the intercellular communication between the different components of the TME modulating tumor response.
- secretory autophagy
- exosomes
- secretion
- melanoma
- tumor microenvironment
- tumor resistance
1. Introduction
Melanoma is a type of skin cancer developing from the melanocytes. Despite being a rare skin cancer, it is the most aggressive due its heterogeneity and high mutational burden [1]. Although several therapies are available for patients, the treatment to advanced melanoma patients is still poor and is frequently associated with side effects and acquired resistance [2,3][2][3]. In this regard, autophagy machinery has been flagged as a resistance mechanism of melanoma cells to alleviate metabolic stress induced by chemotherapeutic drugs; also, it has been considered an important component in melanogenesis and melanoma progression [4,5,6,7][4][5][6][7].
Autophagy is a self-digestion process that contributes to nutrient recycling and quality control by lysosome-dependent degradation of damaged organelles and proteins. In physiological conditions, autophagy is necessary to maintain the cell homeostasis; however, in stress conditions, such as those in which tumor cells are exposed, autophagy is usually induced as a self-protective mechanism. Nevertheless, autophagy has a dynamic role in cancer, being involved both in tumor prevention and promotion [8]. Furthermore, it is established that autophagy has a crucial role within the tumor microenvironment (TME). The TME is a complex piece of cancer, consisting of stroma cells and components of the extracellular matrix, critical to determinate tumor fate. The physiological features of the TME, such as nutrient deprivation, acidity, hypoxia, and inflammation are stressors capable of induce autophagy, which may modulate tumor progression and therapy outcome. Thus, exploring the autophagy system in melanoma TME is extremely important for discovering new therapies and enlightening the mechanisms that regulate tumor malignity. Therefore, besides the classical degradative function, novel autophagy roles were described in the last decade. Recent findings related to autophagy mediating unconventional secretion demonstrate that cancer cells can communicate with the surrounding cell by an autophagy-dependent secretion named secretory autophagy (SA), which changes functions of immune cells, induces drug resistance, and drives an invasive behavior [9,10,11][9][10][11]. Interestingly, this new secretory function of autophagy reveals an overlap between autophagy and the vesicular membrane trafficking.
2. Secretory Mechanisms: A Complex Network for Cargo Release
Living cells make use of secretion pathways to deliver intracellular components to the extracellular milieu. Secreted factors released outside can be part of a clearance mechanism or also mediators of intercellular communication, both in normal and cancer cells, playing physiological and pathological roles, respectively. One example of such a mechanism in normal cells is the transfer of melanosome vesicles from melanocytes, cells from which melanoma derives [12]. Considering this primary function of melanocytes and knowing the stromal mimicry by melanoma cells, here wthe researchers emphasize and explore the influence of the secretory mechanisms used by melanoma cells and their impact within the TME [13].
2.1. Secretory Autophagy
This process is a non-degradative function of autophagy, where the cells control the release of material to the extracellular environment. Although this mechanism was observed years ago, it is only now well recognized as a novel secretory pathway. In the last few years, many studies uncovered the molecular machinery involved in secretory autophagy; however, several issues have not been explained yet, in part due to the tight proximity with the classical autophagy and the endosomal machinery. However, some authors defend that as SA it is not a degradation mechanism, it should not be termed as an autophagy (“self eating”) process, but the participation of several classical autophagy components in SA route raise questions about whether it can be considered an entire different pathway or just an alternative route. The same difficulty emerges from SA and exosomes, which are a subtype of extracellular vesicles (EVs) secreted by cells that will be better discussed below. Since they have common proteins involved in their biogenesis, doubts about whether they represent different branches of a single mechanism rather than two independent pathways are still not solved [14,15][14][15].
Secretory autophagy (SA) is an independent ER-Golgi route that supports the traffic of a growing list of proteins that use unconventional secretory mechanisms and proteins that are redirecting to this pathway, from classical to unconventional secretion, under stress conditions [16]. One of the first molecules secreted by the secretory autophagy pathway was the pro-inflammatory cytokine IL-1β [17]. Because SA secretes molecules like IL-1β, IL-18 and HMGB1, initially it was related to inflammatory processes; however, later studies described other substrates, such as Annexin-I and Galectin-3 [18].
Briefly, in this process the cargo is not ubiquitinated to be degraded in the lysosomes; instead, the proteins in the autophagosomes are secreted out after their fusion with the multivesicular bodies (MVBs) to form amphisomes, which are then fused to secretory lysosomes or direct to the plasma membrane [19,20][19][20]. Notice that degradative and secretory autophagy share several molecules and events, such as the formation of the intermediate organelle and the amphisome, before the fusion with the lysosomes or the plasma membrane, respectively.
SA requires several components of the autophagy system, such as ATGs and SNAREs, which are involved in the fusion membrane, and the Rab-GTPase family proteins related to vesicular trafficking. The first studies examining the source of the membranes involved in the assemblage of secretory autophagosomes were conducted in yeast upon starvation; the authors indicated the association of membrane microdomains rich in PI3P, called the compartment for unconventional protein secretion (CUPS), with the initiation of autophagosome formation [21]. In mammals, the initial phase of the SA remains unclear, but it is supposed that the biogenesis of secretory autophagosomes occurs from an omegasome-like structure, equivalent to CUSP (Figure 1). In this step, the participation of GRASP65 and GRAP55 (Golgi Reassembly Stacking Proteins) was found [22,23][22][23]. In addition, a few authors demonstrated that multiple autophagy proteins, such as ATG7, ATG3, ATG5, ATG12, ATG16LI, and LC3II, play a significant role in autophagy secretion [24]. One example is the study of Young and Cols that noted a link between autophagy and senescence describing autophagy activation as an effector mechanism of the senescence responsible for the senescence-associated secretion of IL-6 and IL-8. As the mRNA levels of both interleukins were higher in Atg5/7 knockdown cells, the secretion occurs by autophagy-dependent posttranslational mechanism [25]. As well, autophagy-competent cancer cells treated with chemotherapy induced an immunogenic ATP release, unlike that observed in Atg5/7 knockdown cells [11]. In another work, the interaction between the ESCRT-associated protein Alix and the Atg12-Atg3 complex were necessary to maintain MVBs morphology and vesicular traffic as well as the exosome biogenesis [26].
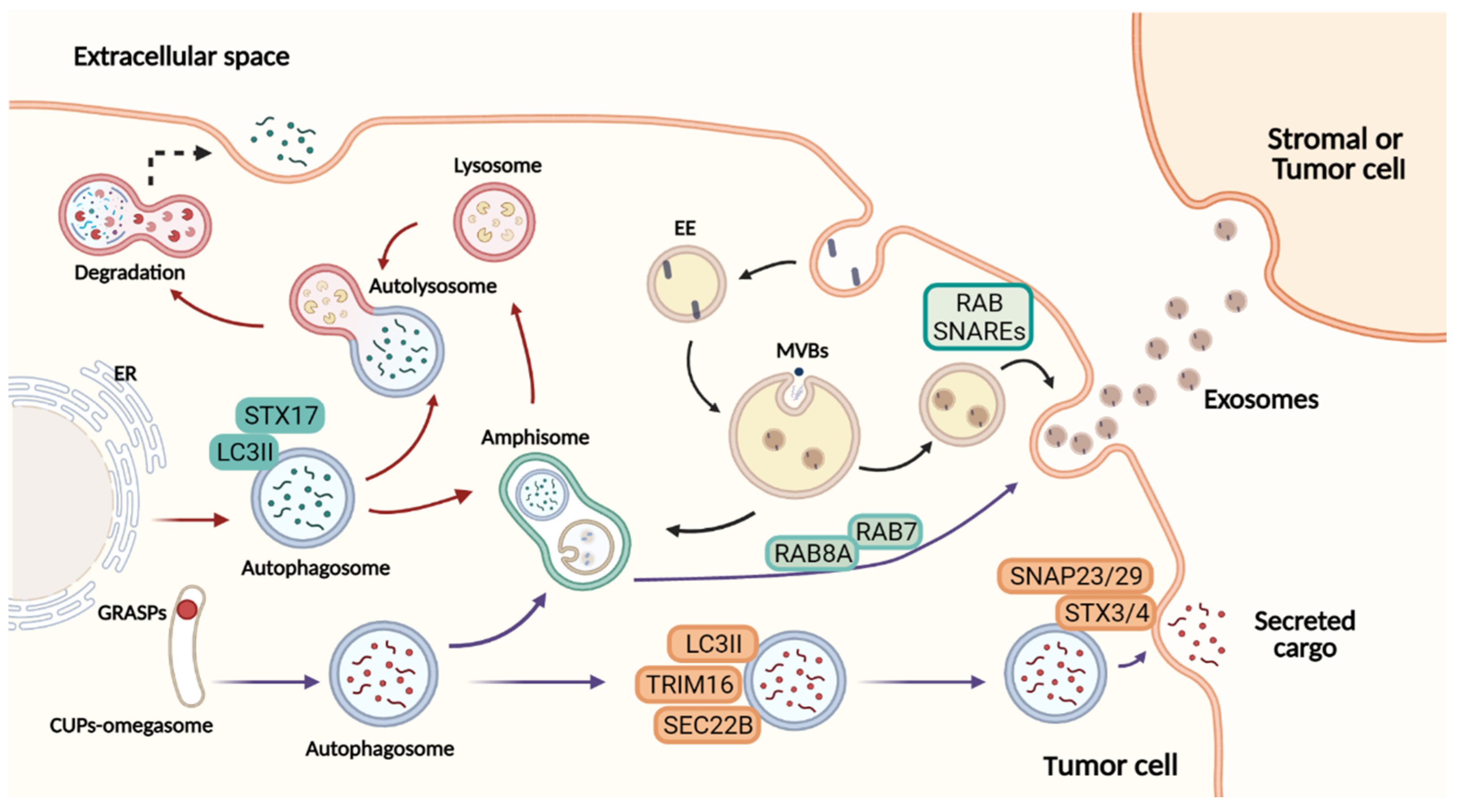

Figure 1. Overview of autophagy-dependent secretory pathways and exosome release. Figure created with BioRender.com.At the ER exit sites, omegasomes contribute to the formation of degradative autophagosomes. LC3II is recruited to the autophagosome membranes, where it mediates membrane expansion. Autophagosomes fuse with lysosomes to form autolysosomes, and their lysosomal hydrolases degrade the cytosolic cargo selected. In a secretory autophagy, the autophagosome is formed by an omegasome-like structure similar to CUPs, near to the ER exit sites. This autophagosome requires TRIM16 for cargo selection, and SEC22B to be addressed to the plasma membrane completing the fusion via complementary SNAREs. Both in the degrading and the secretory pathway, the autophagosome may fuse with the MVBs, generating amphisomes that can either fuse with lysosomes and degrade their content or fuse with the plasma membrane secreting cargo to the extracellular space. Exosome biogenesis initiates from invaginations of the plasma membrane to form early endosomes followed by maturation and inward membrane invaginations to generate ILVs. Further, the MVBs (later endosomes) will fuse with the plasma membrane to extracellularly release the cytosolic components of ILVs as exosomes. The cargo secreted to the extracellular milieu contributes with the transference of several molecules upon the uptake by recipient cells. In this manner, secretory autophagy and exosomes orchestrate multiple systemic processes. Red arrows: degradative autophagy. Purple arrows: secretory autophagy. Black arrows: exosome biogenesis. ER: endoplasmic reticulum. EE: early endosome. MBVs: multivesicular bodies. ILVs: intraluminal vesicles.
How secretory autophagy guides the autophagosome to the plasma membrane is uncertain but latest studies reported some key elements. Investigating protein secretion upon the lysosomal damage model, Kimura et al. found specialized cargo receptors of the TRIM family. Among them, TRIM16 proved to be necessary for the secretion of IL-1β in cooperation with an R-SNARE, called Sec22B. Then, the autophagosome is addressed to the plasma membrane where it finds Qa-SNARES, syntaxin 3 and 4, and Qbc-SNAREs to form a SNARE complex responsible for the membrane fusion (Figure 1) [27]. Nevertheless, the autophagosome can follow a different path to fuse with the MVBs and give origin to the amphisomes. The latter can be degraded by fusion with lysosomes or released to the extracellular milieu [28]. Due to a hybrid origin, the amphisomes carry classical autophagy markers, such as ATG5, ATG16L1, and LC3 and endosomal markers such as Rab7, Rab11, Rab27, and Rab35 [14]. This feature indicates a connection between both pathways.
2.2. Branches of the Secretory Autophagy System
Recent evidence showed a novel mechanism, where LC3 is implicated in the loading of RNA-binding proteins (RBPs) and small non-coding RNAs within EVs. This process, named LC3-dependent EV loading and secretion (LDELS), requires the LC3 conjugation machinery; however, no other ATGs are involved in degradative autophagy, representing an alternate route for the EVs cargo secretion. In LDELS, LC3 and vesicular cargo are delivered to the limiting membrane of MVBs, where the cytosolic cargo undergo budding, generating intraluminal vesicles (ILVs) within the MVBs that subsequently are released as extracellular vesicles. Importantly, when these EVs were isolated and fractionated, it was observed that LC3 was in the lumen of EVs, thus corroborating that LC3 is secreted within EVs [29]. Afterwards, another elegant study from the same group revealed a different secretory autophagy route in response to the endolysosome inhibition. They showed that the inhibition of lysosome acidification by bafilomycin A1 activates secretory autophagy of autophagy elements and cargo receptors in EVs fractions, both in vitro and in vivo [30]. In contrast to LDELS, the cargo is not loaded selectively into EVs, being exposed to proteolysis instead. Thus, these proteins are released in association with EVs, but as a fraction of nanoparticles, named extracellular vesicles and particles (EVP) [31]. Thus, this secretory pathway is dependent on several ATG as well as endosomal markers, such as Rab27a, which are responsible for the exocytosis of EVPs. In addition, the impairment of the degradative autophagy induced EVP-associated secretion maintaining cell protein homeostasis. This suggests a regulation between secretory and degradative autophagy. Otherwise, this regulation is also demonstrated in other studies; Kraya and Cols described that high autophagy levels in melanoma cells impact the level of secreted proteins, increasing cellular secretion [9]. Interestingly, these authors also observed higher levels of autophagy and protein secretion, such as CXCL8 and IL-1β, in metastatic melanomas in comparison to cells derived from primary lesions. CXCL8 has been described as an important regulator of growth, angiogenesis, migration, and metastasis in melanomas through its binding to CXCR1 and CXCR2 receptors [32,33,34][32][33][34]. Consistently, Scheibenbogen and co-authors (1995) demonstrated that higher levels of CXCL8 secreted by melanoma cells correlate with poor prognosis. Concerning IL1-β, using a pre-clinical model, Tengesdal et al. (2021) demonstrated that IL-1β induces IL-6/STAT3 activation in melanoma, inducing an immunosuppressive environment that favors tumor growth. Then, based on these findings, one might speculate that along melanoma progression, tumor sub clones demonstrating increased levels of SA and EVPs are selected due to their ability in communicating with stromal cells to imprint a permissive microenvironment for tumor growth and metastasis, highlighting the importance of unconventional secretory pathways as an important hallmark in melanoma evolution [35,36][35][36].
2.3. Crosstalk between Extracellular Vesicles and the Autophagy Pathway
EVs are a heterogeneous population of spheric lipid bilayer vesicles, which are secreted by most cells to biological fluids playing key roles in physiological and pathological processes. EVs contain several molecules, such as proteins, lipids, non-coding RNAs, mRNA, DNA, and cytokines protected by a double membrane. Thus, the main attribute of EVs is related to its function as mediators of intercellular communication. There are different classes of EVs, classified based on size, biogenesis, content, and function. The two groups best characterized and most studied of EVs are: microvesicles (100–1000 nm) and exosomes (30–150 nm) [37]. Owing to their endosomal origin, the exosomes share some molecules and events with SA. During the exosome generation, the cargo is sorted to invaginations of the endosomal membrane establishing ILVs within the MBVs, which fuse with the plasma membrane to release the intraluminal vesicles to the extracellular compartment as exosomes (Figure 1) [38]. The exosomes convey different kinds of molecular messengers between cells contributing with the regulation of cellular phenotypes. Then, in cancer, the interest of the tumor-derived extracellular vesicles (TEVs) has grown due to its crucial signaling role between tumor cells and the TME supporting tumor progression, tumor resistance, and their potential as diagnostic and prognostic biomarkers.
Considering the above data and the importance of the intercellular communication into the TME, recent studies try to shed light on the importance of vesicular secretion programs in melanocytes and melanoma cells. Next, wthe researchers further discuss the conjunction of these pathways in melanocytes biology and in the context of melanoma tumor progression, drug response, and recurrence.
3. Role of Secretory Autophagy and Exosomes in Melanocytes Biology
Although they are still described as different cellular secretory routes, the similarities between autophagy and exosomes have been reported by some groups and, in fact, are also reflected in their history. In the past years, for example, both processes were seen as a way for cells to get rid of waste or undesirable molecules [39]. However, over the years, the understanding about their biology revealed that both cellular processes are also involved in homeostasis maintenance and cell-to-cell communication. Regarding the common molecules, HSPA8, HSP90AA1, VCP, Rab7A, Rab8a, GRASPs, LC3, ESCRTs, SNAREs, and several Atg proteins are the main ones described [17,40,41,42][17][40][41][42].
Autophagy and exosomes machinery have been reported to play an important role in skin melanocytes. These cells are the ones responsible for pigment production through the conversion of tyrosine into melanin, in a multi-step process that relies on intense intracellular vesicle trafficking, which culminates in melanosome formation. Exosome biogenesis-related molecules as well as proteins of the autophagic machinery are involved in this vesicular movement inside melanocytes in a very coordinated way, which guarantees the production and packing of melanin in melanosomes and their transfer to keratinocytes [43,44,45,46][43][44][45][46]. Furthermore, it is known that melanocytes are often exposed to stressful environmental conditions, such as UV irradiation. In this scenario, the induction of autophagy and extracellular vesicle release is reported in human cutaneous melanocytes. Shen and co-authors (2020) observed an increase in exosome release by human melanocytes after UVB exposure [47]. Regarding their cargo, an enrichment in miR-320d, miR-4488, and miR-7740 was detected by the authors, suggesting that gene expression might be affected in recipient cells. Interestingly, in the same year, Sha and Cols also demonstrated an increase in exosome secretion by melanocytes in response to UV [48]. Juvenil human melanocytes entered a premature senescent-like state, which was accompanied by a decrease in most NER (nucleotide excision repair) genes after UV exposure. The exosomes released under this condition exhibited a distinct microRNA cargo known to target genes related to senescent phenotype, and the authors suggested that these nanostructures are responsible for the senescent phenotype in irradiated melanocytes, which might favor the malignant transformation of melanocytes for leaving these cells vulnerable to irreversible DNA lesions.
In the same way, autophagy has been reported as an important mechanism for skin homeostasis and, consequently, is involved in processes related to skin aging, senescence, and UV response. Regarding the latest processes, in 2016, Wäster and colleagues demonstrated that UVA induced plasma membrane damage in melanocytes and lysosomal exocytosis is involved in its repair [49]. In relation to senescence, using Atg7-deficient skin melanocytes, Zhang and colleagues observed the induction of premature senescence in these cells, characterized by the accumulation of p62, up-regulation of NFR2 (nuclear factor E2–related factor 2), and increase in oxidative stress [50]. The induction of both autophagy and senescence phenotype in stress conditions, such as DNA damage and oxidative stress, suggest a link between them. Supporting this, Ni and Cols demonstrated an increase in inflammatory chemokines and cytokines secretion by Atg7-deficient melanocytes, which was associated with the senescence-associated secretory phenotype (SASP) [51]. In addition, melanocytes transduced with BRAF oncogene, representing an early-stage pathogenesis melanoma model, demonstrated that the oncogene-induced proliferation can be regulated by ATG5 expression; hence, the down-regulation of ATG5 lead to low autophagy levels allowing proliferation and preventing senescence [52]. Collectively, these studies highlight the involvement of the vesicular secretory pathways as mediators of melanocyte stress response; however, it is still not clear whether these processes act in synergism or even if they indeed constitute the same cellular pathway evoked in response to a stress condition. From outhe researchers point of view, in any of these scenarios, the activation of autophagy and/or exosome release represents an effective way to survive stressful situations. WeThe researchers believe that this strategy is also employed by malignant melanoma cells to bypass the cytotoxicity imposed by oncological therapies, as discussed below.
4. Melanoma Progression, Tumor Microenvironment, and Autophagy
The environment around the tumor is a complex network of stromal cells, including endothelial cells, fibroblasts, and immune cells as well as the non-cellular components, such as the extracellular matrix and secreted factors, which are all important to stimulate tumor heterogeneity. Each element of the TME has essential roles to control several stages of tumorigenesis, such as tumor growth, invasion, metastasis, and neovascularization. Among them, cancer cells and tumor-associated stromal cells are crucial in altering the equilibrium of extracellular matrix remodeling events, providing a dynamic niche capable of sustaining malignant transformation [53]. In turn, the extracellular matrix impacts proliferation, mobility, and tumor vascularization. In the latest, changes in tissue tumor architecture, caused by the overgrowth of cancer cells, aid the establishment of the tumor hypoxia state, which ultimately induces a switch to the angiogenic phenotype. Such events lead to the sprouting of new blood vessels vital to deliver sufficient oxygen and nutrients to tumor survival [54,55][54][55]. Above all, the autophagy secretion process has emerged as a regulator of this phenomenon. For instance, the secretion of different cytokines, such as VEGF-A and IL-8 by melanoma cells, trigger pro-angiogenic signaling in surrounding endothelial cells and prompting an angiogenesis switch, which is correlated with the transition of melanocytic lesions to vertical growth phase—a stage with a higher capacity to metastasize [17,56,57][17][56][57].
The interplay between TME components and secretory autophagy are also crucially involved in local invasion and metastasis, important features in high resistant and metastatic tumors, such as malignant melanoma. In this context, it was demonstrated that MMP-9 and MMP-2 in melanoma patients are key players in extracellular matrix degradation and drivers of cancer cell spreading and metastasis [58,59][58][59]. These peptidases are regulated by different molecules, such as the angiogenic factor IL-8 in an autophagy-dependent secretion manner [33], reinforcing the notion that SA establishes an effective communication in the TME. Moreover, another way that autophagy contributes to TME reprogramming is through the regulation of immune cells. Based on the immunogenic phenotype of melanomas characterized by the infiltration of different immune cells, the activity of the autophagy pathway in this scenario is meaningful. Inhibition of autophagy in melanoma cells, for example, promotes NK (natural killer) cells infiltration and activation against the malignant cells. Interestingly, targeting the autophagy process in MDSC (myeloid-suppressor derived cells) reprograms these cells towards an anti-tumoral phenotype. On the other hand, the secretion of diverse factors into the TME through SA enables the induction of immune evasion and immunosuppression, affecting therapy responses and contributing resistance (reviewed in [60,61][60][61]), as better discussed in the next section. Then, taken together, these studies highlight the oncogenic role of autophagy in the TME in the context of tumor cells as well as stromal cells, and, in fact, wthe researchers imagine that its blockade specifically in immune cells might improve immunotherapy response and provide clinical benefits to melanoma patients.
References
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358.
- Haas, L.; Elewaut, A.; Gerard, C.L.; Umkehrer, C.; Leiendecker, L.; Pedersen, M.; Krecioch, I.; Hoffmann, D.; Novatchkova, M.; Kuttke, M.; et al. Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat. Cancer 2021, 2, 693–708.
- Patel, M.; Eckburg, A.; Gantiwala, S.; Hart, Z.; Dein, J.; Lam, K.; Puri, N. Resistance to Molecularly Targeted Therapies in Melanoma. Cancers 2021, 13, 1115.
- Verykiou, S.; Alexander, M.; Edwards, N.; Plummer, R.; Chaudhry, B.; Lovat, P.; Hill, D.S. Harnessing autophagy to overcome mitogen-activated protein kinase kinase inhibitor-induced resistance in metastatic melanoma. Br. J. Dermatol. 2019, 180, 346–356.
- Ma, X.-H.; Piao, S.-F.; Dey, S.; McAfee, Q.; Karakousis, G.; Villanueva, J.; Hart, L.S.; Levi, S.; Hu, J.; Zhang, G.; et al. Targeting ER stress–induced autophagy overcomes BRAF inhibitor resistance in melanoma. J. Clin. Investig. 2014, 124, 1406–1417.
- Yang, Y.; Jang, G.-B.; Yang, X.; Wang, Q.; He, S.; Li, S.; Quach, C.; Zhao, S.; Li, F.; Yuan, Z.; et al. Central role of autophagic UVRAG in melanogenesis and the suntan response. Proc. Natl. Acad. Sci. USA 2018, 115, E7728–E7737.
- Möller, K.; Sigurbjornsdottir, S.; Arnthorsson, A.O.; Pogenberg, V.; Dilshat, R.; Fock, V.; Brynjolfsdottir, S.H.; Bindesboll, C.; Bessadottir, M.; Ogmundsdottir, H.M.; et al. MITF has a central role in regulating starvation-induced autophagy in melanoma. Sci. Rep. 2019, 9, 1055.
- Katheder, N.; Khezri, R.; Ofarrell, F.; Schultz, S.W.; Jain, A.; Rahman, M.M.; Schink, K.O.; Theodossiou, T.A.; Johansen, T.; Juhasz, G.; et al. Microenvironmental autophagy promotes tumour growth. Nature 2017, 541, 417–420.
- Kraya, A.A.; Piao, S.; Xu, X.; Zhang, G.; Herlyn, M.; Gimotty, P.; Levine, B.; Amaravadi, R.K.; Speicher, D.W. Identification of secreted proteins that reflect autophagy dynamics within tumor cells. Autophagy 2015, 11, 60–74.
- Maycotte, P.; Jones, K.L.; Goodall, M.L.; Thorburn, J.; Thorburn, A. Autophagy Supports Breast Cancer Stem Cell Maintenance by Regulating IL6 Secretion. Mol. Cancer Res. 2015, 13, 651–658.
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G.; et al. Autophagy-Dependent Anticancer Immune Responses Induced by Chemotherapeutic Agents in Mice. Science 2011, 334, 1573–1577.
- Moreiras, H.; Seabra, M.; Barral, D. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466.
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nat. Commun. 2020, 11, 2858.
- Chen, Y.-D.; Fang, Y.-T.; Cheng, Y.-L.; Lin, C.-F.; Hsu, L.-J.; Wang, S.-Y.; Anderson, R.; Chang, C.-P.; Lin, Y.-S. Exophagy of annexin A2 via RAB11, RAB8A and RAB27A in IFN-γ-stimulated lung epithelial cells. Sci. Rep. 2017, 7, 5676.
- Leidal, A.M.; Debnath, J. Emerging roles for the autophagy machinery in extracellular vesicle biogenesis and secretion. FASEB BioAdv. 2021, 3, 377–386.
- Gee, H.Y.; Noh, S.H.; Tang, B.L.; Kim, K.H.; Lee, M.G. Rescue of ΔF508-CFTR Trafficking via a GRASP-Dependent Unconventional Secretion Pathway. Cell 2011, 146, 746–760.
- Dupont, N.; Jiang, S.; Pilli, M.; Ornatowski, W.; Bhattacharya, D.; Deretic, V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 2011, 30, 4701–4711.
- Popa, S.; Stewart, S.E.; Moreau, K. Unconventional secretion of annexins and galectins. Semin. Cell Dev. Biol. 2018, 83, 42–50.
- Takenouchi, T.; Nakai, M.; Iwamaru, Y.; Sugama, S.; Tsukimoto, M.; Fujita, M.; Wei, J.; Sekigawa, A.; Sato, M.; Kojima, S.; et al. The Activation of P2X7 Receptor Impairs Lysosomal Functions and Stimulates the Release of Autophagolysosomes in Microglial Cells. J. Immunol. 2009, 182, 2051–2062.
- Ponpuak, M.; Mandell, M.; Kimura, T.; Chauhan, S.; Cleyrat, C.; Deretic, V. Secretory autophagy. Curr. Opin. Cell Biol. 2015, 35, 106–116.
- Bruns, C.; McCaffery, J.M.; Curwin, A.; Duran, J.M.; Malhotra, V. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J. Cell Biol. 2011, 195, 979–992.
- Duran, J.M.; Anjard, C.; Stefan, C.; Loomis, W.F.; Malhotra, V. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 2010, 188, 527–536.
- Ahat, E.; Li, J.; Wang, Y. New Insights into the Golgi Stacking Proteins. Front. Cell Dev. Biol. 2019, 7, 131.
- Zhang, M.; Kenny, S.J.; Ge, L.; Xu, K.; Schekman, R. Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife 2015, 4, e11205.
- Young, A.R.; Narita, M.; Ferreira, M.; Kirschner, K.; Sadaie, M.; Darot, J.F.; Tavaré, S.; Arakawa, S.; Shimizu, S.; Watt, F.M.; et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009, 23, 798–803.
- Murrow, L.; Malhotra, R.; Debnath, J. ATG12–ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 2015, 17, 300–310.
- Kimura, T.; Jia, J.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; Farzam, F.; et al. Dedicated SNARE s and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017, 36, 42–60.
- Shibutani, S.T.; Yoshimori, T. A current perspective of autophagosome biogenesis. Cell Res. 2014, 24, 58–68.
- Leidal, A.M.; Huang, H.H.; Marsh, T.; Solvik, T.; Zhang, D.; Ye, J.; Kai, F.; Goldsmith, J.; Liu, J.Y.; Huang, Y.-H.; et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 2020, 22, 187–199.
- Solvik, T.A.; Nguyen, T.A.; Lin, Y.-H.T.; Marsh, T.; Huang, E.J.; Wiita, A.P.; Debnath, J.; Leidal, A.M. Autophagy cargo receptors are secreted via extracellular vesicles and particles in response to endolysosomal inhibition or impaired autophagosome maturation. bioRxiv 2021.
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343.
- Singh, R.K.; Varney, M.L. Regulation of interleukin 8 expression in human malignant melanoma cells. Cancer Res. 1998, 58, 1532–1537.
- Luca, M.; Huang, S.; Gershenwald, J.E.; Singh, R.K.; Reich, R.; Bar-Eli, M. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am. J. Pathol. 1997, 151, 1105–1113.
- Singh, S.; Singh, A.P.; Sharma, B.; Owen, L.B.; Singh, R.K. CXCL8 and its cognate receptors in melanoma progression and metastasis. Futur. Oncol. 2010, 6, 111–116.
- Scheibenbogen, C.; Möhler, T.; Haefele, J.; Hunstein, W.; Keilholz, U. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995, 5, 179–182.
- Tengesdal, I.W.; Dinarello, A.; Powers, N.E.; Burchill, M.A.; Joosten, L.A.B.; Marchetti, C.; Dinarello, C.A. Tumor NLRP3-Derived IL-1β Drives the IL-6/STAT3 Axis Resulting in Sustained MDSC-Mediated Immunosuppression. Front. Immunol. 2021, 12, 3439.
- Möller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 2020, 20, 697–709.
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 2003505.
- Gudbergsson, J.M.; Johnsen, K.B. Exosomes and autophagy: Rekindling the vesicular waste hypothesis. J. Cell Commun. Signal. 2019, 13, 443–450.
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012, 10, e1001450.
- Patel, K.K.; Miyoshi, H.; Beatty, W.L.; Head, R.D.; Malvin, N.P.; Cadwell, K.; Guan, J.-L.; Saitoh, T.; Akira, S.; O Seglen, P.; et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J. 2013, 32, 3130–3144.
- Galluzzi, L.; Green, D.R. Autophagy-Independent Functions of the Autophagy Machinery. Cell 2019, 177, 1682–1699.
- Jimbow, K.; Hua, C.; Gomez, P.F.; Hirosaki, K.; Shinoda, K.; Salopek, T.G.; Matsusaka, H.; Jin, H.-Y.; Yamashita, T. Intracellular Vesicular Trafficking of Tyrosinase Gene Family Protein in Eu- and Pheomelanosome Biogenesis. Pigment. Cell Res. 2000, 13, 110–117.
- Sitaram, A.; Marks, M.S. Mechanisms of Protein Delivery to Melanosomes in Pigment Cells. Physiology 2012, 27, 85–99.
- Ramkumar, A.; Murthy, D.; Raja, D.A.; Singh, A.; Krishnan, A.; Khanna, S.; Vats, A.; Thukral, L.; Sharma, P.; Sivasubbu, S.; et al. Classical autophagy proteins LC3B and ATG4B facilitate melanosome movement on cytoskeletal tracks. Autophagy 2017, 13, 1331–1347.
- Ohbayashi, N.; Fukuda, M. SNARE dynamics during melanosome maturation. Biochem. Soc. Trans. 2018, 46, 911–917.
- Shen, Z.; Sun, J.; Shao, J.; Xu, J. Ultraviolet B irradiation enhances the secretion of exosomes by human primary melanocytes and changes their exosomal miRNA profile. PLoS ONE 2020, 15, e0237023.
- Sha, J.; Arbesman, J.; Harter, M.L. Premature senescence in human melanocytes after exposure to solar UVR: An exosome and UV-miRNA connection. Pigment. Cell Melanoma Res. 2020, 33, 671–684.
- Wäster, P.; Eriksson, I.; Vainikka, L.; Rosdahl, I.; Öllinger, K. Extracellular vesicles are transferred from melanocytes to keratinocytes after UVA irradiation. Sci. Rep. 2016, 6, 27890.
- Zhang, C.-F.; Gruber, F.; Ni, C.; Mildner, M.; Koenig, U.; Karner, S.; Barresi, C.; Rossiter, H.; Narzt, M.-S.; Nagelreiter, I.M.; et al. Suppression of Autophagy Dysregulates the Antioxidant Response and Causes Premature Senescence of Melanocytes. J. Investig. Dermatol. 2015, 135, 1348–1357.
- Ni, C.; Narzt, M.-S.; Nagelreiter, I.M.; Zhang, C.F.; Larue, L.; Rossiter, H.; Grillari, J.; Tschachler, E.; Gruber, F. Autophagy deficient melanocytes display a senescence associated secretory phenotype that includes oxidized lipid mediators. Int. J. Biochem. Cell Biol. 2016, 81, 375–382.
- Liu, H.; He, Z.; Von Rütte, T.; Yousefi, S.; Hunger, R.E.; Simon, H.-U. Down-Regulation of Autophagy-Related Protein 5 (ATG5) Contributes to the Pathogenesis of Early-Stage Cutaneous Melanoma. Sci. Transl. Med. 2013, 5, 202ra123.
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120.
- Siemann, D.W.; Horsman, M. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol. Ther. 2015, 153, 107–124.
- Maes, H.; Olmeda, D.; Soengas, M.S.; Agostinis, P. Vesicular trafficking mechanisms in endothelial cells as modulators of the tumor vasculature and targets of antiangiogenic therapies. FEBS J. 2015, 283, 25–38.
- Thorburn, J.; Horita, H.; Redzic, J.; Hansen, K.; E Frankel, A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2008, 16, 175–183.
- Damsky, J.W.E.; Rosenbaum, L.E.; Bosenberg, M. Decoding Melanoma Metastasis. Cancers 2010, 3, 126–163.
- Pandita, A.; Ekstrand, M.; Bjursten, S.; Zhao, Z.; Fogelstrand, P.; Le Gal, K.; Ny, L.; Bergo, M.O.; Karlsson, J.; Nilsson, J.A.; et al. Intussusceptive Angiogenesis in Human Metastatic Malignant Melanoma. Am. J. Pathol. 2021, 191, 2023–2038.
- Napoli, S.; Scuderi, C.; Gattuso, G.; Di Bella, V.; Candido, S.; Basile, M.S.; Libra, M.; Falzone, L. Functional Roles of Matrix Metalloproteinases and Their Inhibitors in Melanoma. Cells 2020, 9, 1151.
- Liu, D.; Yang, X.; Wu, X. Tumor Immune Microenvironment Characterization Identifies Prognosis and Immunotherapy-Related Gene Signatures in Melanoma. Front. Immunol. 2021, 12, 663495.
- Di Leo, L.; Bodemeyer, V.; De Zio, D. The Complex Role of Autophagy in Melanoma Evolution: New Perspectives from Mouse Models. Front. Oncol. 2020, 9, 1506.
More
