You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Vicky Zhou and Version 2 by Vicky Zhou.
It is important to rear insects, whether on a small scale for research or a large scale for mass rearing, for use in biological control (BC) programs with macro-organisms.
- sustainability
- agriculture 4.0
- eco-friendly technology
1. Introduction
Brazil is a leader in tropical agriculture, with a forecasted production of 289.7 million tons of agricultural commodities in 2020–2021, reached through technology developed in this country [1]. The mindset of using agrochemicals for pest control prevailed among Brazilian farmers for a long period, although Brazil imported the first natural enemy in 1921 for use in a classical biological control (BC) program [2][3]. This first program used the species Encarsia berlesei Howard (Hym.: Aphelinidae, then assigned to the genus Prospaltella), imported from the USA, to control Pseudaulacaspis pentagona (Targioni) (Hem.: Diaspididae) on peaches. No control resulted, due to the lack of techniques for rearing the pest and the imported natural enemy, a failure that continued until 1940, for other imported species, for the same reason [2]. Biological control in Brazil advanced with the establishment of graduate programs in the 1960s, coincident with the establishment of the Entomological Society of Brazil in 1972. BC advanced from 1980 to 2010 onward, with extension courses on insect rearing techniques for BC, conducted in different states by the team from the Department of Entomology and Acarology of the University of São Paulo (USP), Luiz de Queiroz College of Agriculture (ESALQ).
In 1976, integrated pest management (IPM) was established by Smith et al. (1976) [4]. These programs require the production of insects for different areas, i.e., for the development of measures to maintain pest numbers below the level of economic damage. Mastery of techniques for insect rearing has led to significant advances in several areas, including bioecology, insecticide selectivity, insect pheromones, pest and natural enemy zoning, resistance management, transgenic plants, molecular studies, insect pathology (micro-organisms), and symbionts, but mainly mass rearing of insects for BC with macroorganisms.
Along with the millions of hectares (ha) treated with micro-organisms in Brazil, certain crops, such as sugarcane, are now being treated with macroorganisms, such as Trichogramma galloi Zucchi (Hym.: Trichogrammatidae) (a native parasitoid) and Cotesia flavipes (Cameron) (Hym.: Braconidae) (an exotic parasitoid), on 3 and 3.5 million ha, respectively. Almost all the parasitoids (90–95%) are released by drones at a cost competitive with chemicals. Nowadays, species of Trichogramma Westwood (Hym.: Trichogrammatidae) are used in varying numbers of 50 to 400 thousand individuals per ha in cotton, corn, avocado, citrus, soybean, tomato, and other crops to control lepidopterans [3]. Use of these tiny wasps is made possible by the production of eggs of their factitious host Anagasta kuehniella Zeller (Lep.: Pyralidae), in quantities of up to 30–40 kg of eggs per day (1 g = 36,000 eggs).
Advances in artificial diets, including multidimensional and nutrigenomic studies, would substantially improve rearing techniques for different groups of Lepidoptera, Coleoptera, and Diptera, and for other orders. An example of advance on the last year, nevertheless without the mentioned technics, is the lyophilized green bean-based diet for the main soybean pest, the brown stink bug Euschistus heros (F.) (Hem.: Pentatomidae), to produce its egg parasitoid, the natural enemy Telenomus podisi Ashmead (Hym.: Scelionidae) [5]. Commercial-scale programs to control citrus pests, using, for example, the egg-parasitoid wasp Ageniaspis citricola Logvinoskaya (Hym.: Encyrtidae) to control the leafminer Phyllocnistis citrella Stainton (Lep.: Gracillariidae) and the ectoparasitoid wasp Tamarixia radiata (Waterston) (Hym.: Eulophidae) to control the psyllid Diaphorina citri Kuwayama (Hem.: Psyllidae), are, or have been, routinely used in citrus production, the latter produced on orange jessamine [Murraya paniculata (L.) (Sap.: Rutacaeae)].
2. Biological Control and Insect Rearing
Based on current knowledge of the development of biological control programs, it is necessary to rear the target pest and its natural enemy, as the “in vitro” rearing studies started in the 1980s, with the presentation in China (Guangzhou) [6] of a diet for Trichogramma dendrolimi Matsumura (Hym.: Trichogrammatidae), did not evolve as expected (Cônsoli and Grenier, 2010) [7]. Although Brazil produced species of Trichogramma (T. galloi, Trichogramma pretiosum Riley, Trichogramma atopovirilia Oatman & Platner) and Habrobracon hebetor (Say) (Hym.: Braconidae), viable rearing “in vitro” on a mass scale was not achieved. Despite advances in the field of nutrigenomics and multidimensional systems for artificial diets [8][9][10], it is still necessary to rear two species of insects, the pest, and its natural enemy.
Considering the procedures for biological control, that is, introduction (classical biological control), conservation (natural or conservative biological control), multiplication (augmentative or applied biological control), and external management (for D. citri, transmitter of HLB in citrus [11]), in every case, it is necessary to rear the natural enemy in the laboratory. Natural enemies are needed in varying numbers, and different life stages may be required for particular BC programs.
In the case of classical biological control, during the initiation of BC as a control method, for lack of rearing techniques, the releases were conducted with small numbers of insects (inoculative releases), which required some time for the population of the natural enemy to increase and consequently served only for perennial or semi-perennial crops. The classic case is Rodolia cardinalis (Mulsant) (Col.: Coccinellidae) (currently the species is referred as Novius cardinalis; however, given the consecration of the fact, the species name Rodolia cardinalis is considered in the present work), used in 1888 to control Icerya purchasi Maskell (Hem.: Monophlebidae), which was brought from Australia, the place of origin of the mealybug that had become a pest of citrus in California. This is the first case of true success of modern BC in the world.
In the case of conservative biological control, in which one attempts to manage the habitat, in order to conserve existing control agents and, if possible, to increase them, the techniques used do not always need large numbers of insects for release. Selective products are used, also taking into account the resistance of pests to applied chemical products (resistance management) and provision of food (pollen) to the adults, for example.
In the case of external management, the foci of contamination of the bacteria that cause “Huanglongbing” (HLB) and are transmitted by D. citri are outside the orchard, because in this case large amounts of insecticides are used in the commercial orchards. Tamarixia radiata, the biological control agent, is being released in numbers of 3200 parasitoids/ha on about 12,000 ha each 15 days. Tamarixia radiata is released in areas at distances of up to 1.5 km outside the commercial orchards, in abandoned orchards (where HLB already occurred), organic areas, areas with M. paniculata (host-plant of the psyllid), and residential backyards.
For multiplication, that is, augmentative or applied biological control, it is necessary to produce millions of insects for release; these are the mass rearing, which involve the production of millions of biological control agents for field releases, with thousands of individuals per ha being released simultaneously. In this case, control is more rapid, more closely resembling the chemical products to which the farmer is accustomed.
Mass rearing began to be referenced in the book by Smith (1966) [12] and the compilation provided in a book by Singh (1977) [13] on artificial diets for insects. Other books on insect rearing techniques followed, such as those of Singh and Moore (1985) [14], Cohen (2003, 2015) [15][16], Schneider (2009) [17], Panizzi and Parra (2012) [18], and Morales-Ramos et al. (2014) [19]. These artificial diets facilitated the development of IPM because they allowed insects to be laboratory-reared for a wider range of studies, including studies of BC. These artificial diets were developed mainly for species of Lepidoptera, Coleoptera, and Diptera (although diets exist for other orders) and presently have the same formulations as the diets from 1970–1980. For example, Parra et al. (2021) [20] studied biological aspects and the spatial distribution of the Spodoptera complex at different temperatures, using the diet of Greene et al. (1976) [21]. Several definitions of mass rearing were compiled by Parra (2008) [22].
Finney and Fisher (1964) [23] defined mass rearing as the economical production of millions of beneficial insects in an assembly line, with the objective of producing the maximum number of fertile females, with minimum man-hours and space, in the shortest time possible, and at low cost. Mackauer (1972) [24] and Chambers (1977) [25] combined the economic with the biological aspect and defined mass rearing as the production of insects capable of reaching objectives such as an acceptable cost/benefit ratio and at least exceeding 10 thousand to 1 million times the mean productivity of native females. Leppla and Adams (1986) [26] described mass rearing as an automated activity in integrated installations, with the objective of producing a relatively large supply of good quality insects for distribution.
Therefore, millions of insects are being produced. Labor represents 70–80% of the cost of production. Problems with the quality of the insects produced, as well as the sanitary problems, increase with the size of rearing systems. Considering the extensive agricultural areas in Brazil (10,000, 50,000, or 100,000 ha planted with the same crop), automation is necessary to produce sufficient insects for release in these areas. Storage of the insects produced in mass rearing systems is essential, so that rearing is not interrupted when the insects are not being released in the field. After storage, they must be of equal quality to wild insects. Mass rearing is the result of upscaling from a smaller rearing system, the so-called research rearing used by researchers in universities and research institutes for basic studies, involving only one or two technicians to conduct it.
Considering the complexity and amplitude of studies necessary for a BC program, such a program must follow a certain logic [27][28]. It is suggested that a BC program follow a sequence of events, as cited by Parra (2021) [29] (Figure 1).
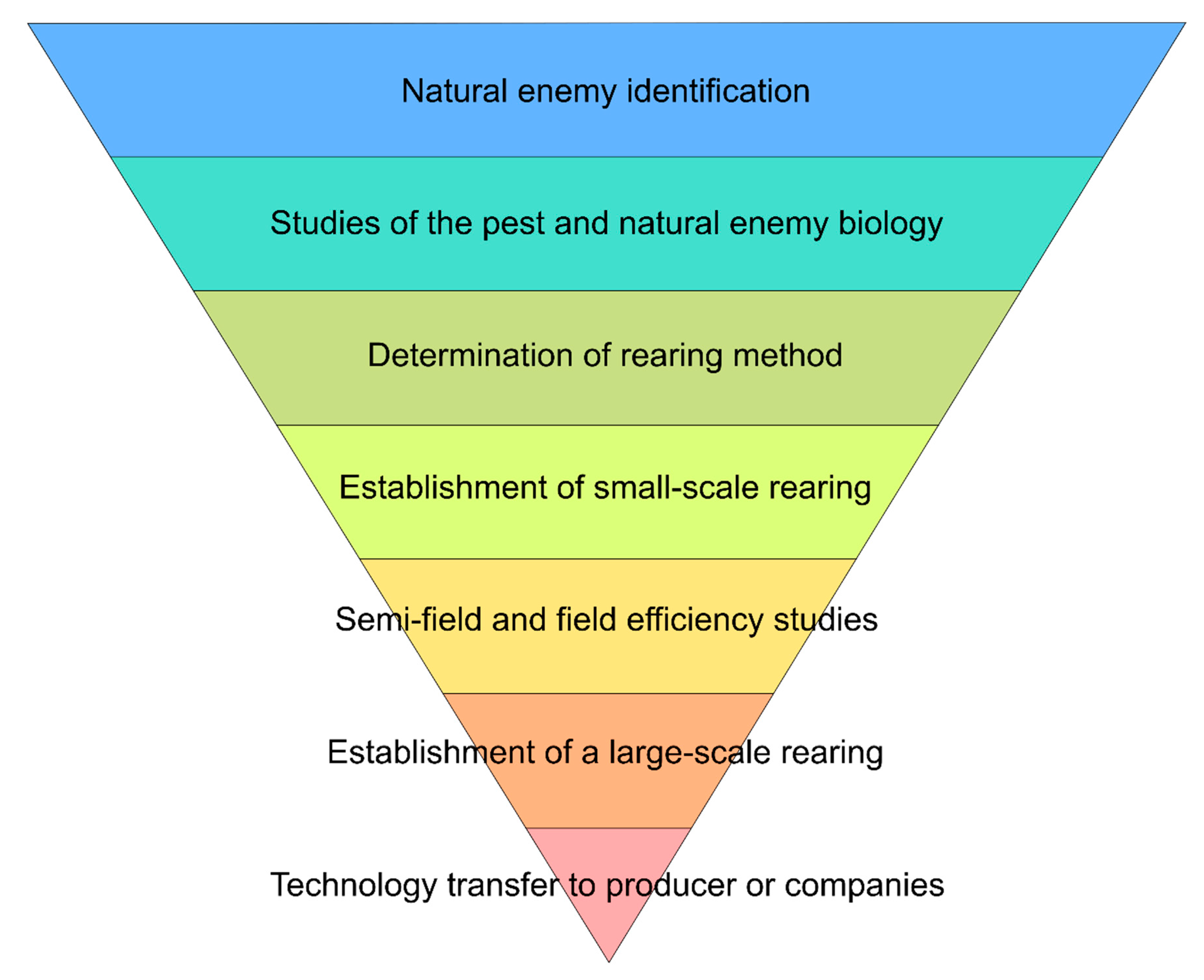
3. Final Remarks
The number of entomologists involved in insect rearing, especially mass rearing, in educational and research institutions is decreasing worldwide, with many specialists moving to other emerging areas, such as biotechnology, robotics, artificial intelligence, nanotechnology, etc. However, the successes in BC described here illustrate the need for a balance. Basic biological studies are needed on both biotic factors (mating, oviposition, adult feeding, and diapause) and abiotic factors (thermal and hygrometric requirements, photoperiod, and CO2). Continual maintenance of laboratory populations is necessary for studies of quality control, chemical selectivity, determination of the release time, number of release points, etc., so that mass rearing and successful biological control can be achieved. Sometimes, the development of an artificial diet, such as the diet for E. heros to allow control with T. podisi [5], can be decisive in a pest control program. In any case, automation of rearing processes will undoubtedly be a huge leap forward in this program, considering that although the 200,000 ha that have been treated seems to be a large area, it is still very small, compared to the 40 million ha of soybeans in Brazil. Rearing of insects, whether pests through artificial diets or on plants, or beneficial insects in factitious or natural hosts, in addition to being essential for BC programs, also has a preponderant role in IPM programs. As already mentioned, rearing of insects enables bioecological, biotechnological, behavioral, resistance-management studies, and selectivity and feasibility studies of entomopathogens. An example of the importance of rearing insects for IPM programs is the case of Gymnandrosoma aurantianum (Lima) (Lep.: Tortricidae), an important citrus pest in Brazil. The diet for this species was developed by Garcia and Parra (1999) [30]. After laboratory rearing methods were established, several studies were carried out, aiming toward the management of this pest; a study of the sexual behavior of the pest, reared on the diet, led to the synthesis of a synthetic sex pheromone, which guided the decision-making of citrus growers [31]. After 10 years of using the sex pheromone, citrus growers in the state of São Paulo avoided losses, estimated at up to 1.3 billion dollars in that period [32]. BC has been increasing in Brazil more than in the rest of the world, and the use of Agriculture 4.0 (precision and digital agriculture) has been decisive for this [33], including pests monitoring [34], the release of natural enemies and automation of mass rearing with modern technologies, such as reflectance [35], robotics, and artificial intelligence. In all cases, insect rearing and mass rearing continue to serve as the foundation for the success of biological control programs.References
- CONAB-Companhia Nacional de Abastecimento. 2021. Available online: https://www.conab.gov.br/info-agro/safras/graos/monitoramento-agricola (accessed on 3 November 2021).
- Parra, J.R.P. Biological control in Brazil: An overview. Sci. Agric. 2014, 71, 420–429.
- Parra, J.R.P.; Coelho, A., Jr. Applied biological control in Brazil: From laboratory assays to field application. J. Insect Sci. 2019, 19, 5.
- Smith, R.F.; Apple, J.L.; Bottrell, D.G. The origins of Integrated Pest Management concepts for agricultural crops. In Integrated Pest Management; Apple, J.L., Smith, R.F., Eds.; Springer: Boston, MA, USA, 1976; pp. 1–16.
- Mendoza, A.C.; da Rocha, A.C.; Parra, J.R. Lyophilized artificial diet for rearing the Neotropical Euschistus heros (Hemiptera: Pentatomidae). J. Insect Sci. 2016, 16, 41.
- Li, L.Y.; Liu, W.H.; Chen, C.S.; Han, S.C.; Shin, J.C.; Du, H.S.; Feng, S.Y. In vitro rearing of Trichogramma spp. and Anastatus sp. in artificial “eggs” and the methods of mass production. Colloq. INRA 1988, 43, 339–352.
- Cônsoli, F.L.; Grenier, S. In vitro rearing of egg parasitoids. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma. Progress in Biological Control; Cônsoli, F.L., Parra, J.R.P., Zucchi, R.A., Eds.; Springer: Dordrecht, Germany, 2009; Volume 9, pp. 293–313.
- Coudron, T.A.; Yocum, G.D.; Brandt, S.L. Nutrigenomics: A case study in the measurement of insect response to nutritional quality. Entomol. Exp. Appl. 2006, 121, 1–14.
- Huynh, M.P.; Meihls, L.N.; Hibbard, B.E.; Lapointe, S.L.; Niedz, R.P.; Ludwick, D.C.; Coudron, T.A. Diet improvement for western corn rootworm (Coleoptera: Chrysomelidae) larvae. PLoS ONE 2017, 12, e0187997.
- Huynh, M.P.; Hibbard, B.E.; Lapointe, S.L.; Niedz, R.P.; French, B.W.; Pereira, A.E.; Finke, D.L.; Shelby, K.S.; Coudron, T.A. Multidimensional approach to formulating a specialized diet for northern corn rootworm larvae. Sci. Rep. 2019, 9, 3709.
- Diniz, A.J.F.; Garcia, A.G.; Alves, G.R.; Reigada, C.; Vieira, J.M.; Parra, J.R.P. The enemy is outside: Releasing the parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in external sources of HLB inocula to control the Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae). Neotrop. Entomol. 2020, 49, 250–257.
- Smith, C.N. Insect Colonization and Mass Production; Academic Press: San Diego, CA, USA, 1966; 612p.
- Singh, P. Artificial Diets for Insects, Mites and Spiders; Plenum Press: Chicago, IL, USA, 1977; 594p.
- Singh, P.; Moore, R.F. Handbook of Insect Rearing, Vol. I and II; Elsevier: Amsterdam, The Netherlands, 1985.
- Cohen, A.C. Insect Diets: Science and Technology, 1st ed.; CRS Press: Cleveland, IL, USA, 2003.
- Cohen, A.C. Insect Diets: Science and Technology, 2nd ed.; CRS Press: Boca Raton, FL, USA, 2015.
- Schneider, J.C. Principles and Procedures for Rearing High Quality Insects; Mississippi State University: Starkville, MS, USA, 2009.
- Panizzi, A.R.; Parra, J.R.P. Insect Bioecology and Nutrition for Integrated Pest Management; CRC Press: New York, NY, USA, 2012; 750p.
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I. Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens; Academic Press: San Diego, CA, USA, 2014; 711p.
- Parra, J.R.P.; Coelho, A., Jr.; Cuervo-Rugno, J.B.; Garcia, A.G.; Moral, R.A.; Specht, A.; Dourado Neto, D. Important pest species of the Spodoptera complex: Biology, thermal requirements and ecological zoning. J. Pest Sci. 2022, 95, 169–186.
- Greene, G.L.; Leppla, N.C.; Dickerson, W.A. Velvetbean caterpillar: A rearing procedure and artificial medium. J. Econ. Entomol. 1976, 69, 487–488.
- Parra, J.R.P. Mass rearing of natural enemies. In Encyclopedia of Entomology, 2nd ed.; Capinera, J.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 4, pp. 2301–2305.
- Finney, G.L.; Fisher, T.W. Culture of entomophagous insects and their hosts. In Biological Control of Pests and Weeds; de Bach, P., Schingler, E.I., Eds.; Chapman & Hall: London, UK, 1964; pp. 328–353.
- Mackauer, M. Genetic aspects of insect production. Entomophaga 1972, 17, 27–48.
- Chambers, D.L. Quality control in mass rearing. Annu. Rev. Entomol. 1977, 22, 289–308.
- Leppla, N.C.; Adams, F. Insect mass-rearing technology. In Principles and Applications; University of Florida: Gainesville, FL, USA, 1986; 20p.
- Ridgway, R.L.; Inscoe, M.N. Mass-reared natural enemies for pest control: Trends and challenges. In Mass-Reared Natural Enemies: Application, Regulation, and Needs; Ridgway, R.L., Hoffmann, M.P., Inscoe, M.N., Glenister, C.S., Eds.; Thomas Say Publications in Entomology: Lanham, MD, USA, 1998; pp. 1–27.
- Parra, J.R.P.; Botelho, P.S.M.; Corrêa-Ferreira, B.S.; Bento, J.M.S. Controle Biológico: Terminologia. In Controle Biológico No Brasil—Parasitóides e Predadores; Parra, J.R.P., Botelho, P.S.M., Corrêa-Ferreira, B.S., Bento, J.M.S., Eds.; Manole: Barueri, Brazil, 2002; pp. 1–13.
- Parra, J.R.P. Elaboração de programas de controle biológico: Uma visão inter e multidisciplinar. In Controle Biológico com Parasitoides e Predadores na Agricultura Brasileira; Parra, J.R.P., Pinto, A.S., Nava, D.E., Oliveira, R.C., Diniz, A.J.F., Eds.; FEALQ: Piracicaba, Brazil, 2021; pp. 39–54.
- Garcia, M.S.; Parra, J.R. Comparison of several artificial diets with different protein sources for massal rearing of Ecdytolopha aurantiana (Lima) (Lepidoptera: Tortricidae). An. Soc. Entomol. Bras. 1999, 28, 219–232.
- Parra, J.R.P.; Bento, J.M.S.; Garcia, M.S.; Yamamoto, P.T.; Vilela, E.F.; Leal, W.S. Development of a control alternative for the citrus fruit borer, Ecdytolopha aurantiana (Lepidoptera, Tortricidae): From basic research to the grower. Rev. Bras. Entomol. 2004, 48, 561–567.
- Bento, J.M.S.; Parra, J.R.P.; de Miranda, S.H.; Adami, A.C.; Vilela, E.F.; Leal, W.S. How much is a pheromone worth? F1000Research 2016, 5, 1763.
- Bolfe, É.L.; Jorge, L.A.D.C.; Sanches, I.D.A.; Luchiari, A., Jr.; da Costa, C.C.; Victoria, D.D.C.; Inamasu, R.Y.; Grego, C.R.; Ferreira, V.R.; Ramirez, A.R. Precision and digital agriculture: Adoption of technologies and perception of Brazilian farmers. Agriculture 2020, 10, 653.
- Nansen, C.; Geremias, L.D.; Xue, Y.; Huang, F.; Parra, J.R. Agricultural case studies of classification accuracy, spectral resolution, and model over-fitting. Appl. Spectrosc. 2013, 67, 1332–1338.
- Nansen, C.; Coelho, A., Jr.; Vieira, J.M.; Parra, J.R.P. Reflectance-based identification of parasitized host eggs and adult Trichogramma specimens. J. Exp. Biol. 2014, 217, 1187–1192.
More
