Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 8 by Bruce Ren and Version 7 by Bruce Ren.
Advances in medical care, improvements in sanitation, and rising living standards contribute to increased life expectancy. Although this reflects positive human development, it also poses new challenges. Among these, reproductive aging is gradually becoming a key health issue because the age of menopause has remained constant at ~50 years, leading women to live longer in suboptimal endocrine conditions. An adequate understanding of ovarian senescence mechanisms is essential to prevent age-related diseases and to promote wellbeing, health, and longevity in women.
- reproductive aging
- senescence
- extracellular matrix
- whole-ovary decellularization
- ECM-based bio-scaffold
- porcine
1. Introduction
In mammals, the female reproductive system is the first to show signs of physiological aging [1], with fertility decline and hormonal dysfunctions that can, in turn, affect overall health. This leads to multiple medical and psychosocial problems, such as osteoporosis, cardiovascular disease, autoimmune disorders, and depression [2][3]. Although medical care advances and rising living standards have contributed to increasing lifespan, the age of menopause has remained constant at the age of ~50 years, leading women to live longer in suboptimal endocrine conditions [4]. Reproductive aging has therefore gradually become a key health issue and an adequate understanding of its mechanisms is essential in order to prevent age-related diseases and to promote health and longevity in women [5].
Aging is generally described as a complex, multifaceted process, characterized by a progressive accumulation of macroscopic and microscopic modifications, accompanied by molecular and cellular damages that can affect organs, tissues, cells, and subcellular organelles, causing severe biological degenerations and a gradual loss of organ functions [6]. Recent studies also suggest that the non-cellular compartment of the tissues, the extracellular matrix (ECM), may play a key role in aging progression [7]. Indeed, during the last years, it has been clearly demonstrated that ECM contributes not only to physical scaffolding and structural support but also provides biochemical and biomechanical stimuli directly influencing cell behaviour [8]. Cells are indeed able to respond to physical and mechanical cues exerted by the surrounding environment modifying their own morphology, polarity, adhesion, migration, growth, gene expression, and functions [9][10][11][12][13]. However, cell ability to sense changes in ECM compliance (mechanosensation) and to transduce these stimuli into biochemical signals (mechanotransduction) are negatively affected by aging progression [6]. In addition, recent studies also suggested that aging may induce critical alterations in ECM composition and organization [14][15], resulting in biomechanical and biochemical ECM property modifications [6][16], that impact cell-to-matrix interactions as well as cell fate and behaviour.
Reproductive biology addressed growing interest in ovarian mechanobiology in the last decade and, in particular, the dynamic reciprocity that exists between ovarian cells and their microenvironment is currently under investigation [17][18]. In this contest, follicles and oocyte developmental quality have been demonstrated to be strongly influenced by biochemical cues [19] as well as by the physical ovarian microenvironment [20][21][22]. However, at present, only scattered information is available in the literature regarding aging mechanobiology of the reproductive tissues, where several aspects still need to be elucidated.
2. Impact of Aging on the Ovarian Extracellular Matrix
2.1. Macroscopic and Microscopic Analysis of Young and Aged Ovaries
Macroscopic evaluation revealed the typical morphology of young and adult ovaries with numerous follicles ranging from 3- to 8- mm in diameter (Figure 1a). As expected, smaller follicle sizes, 3- 4- mm, were more abundant than larger ones (5- 8- mm) in both young and aged ovaries. However, the rate of 3- 4-mm follicles was higher in young ovaries, while a higher proportion of 5- 8-mm follicles was found in aged ovaries (Figure 1b).
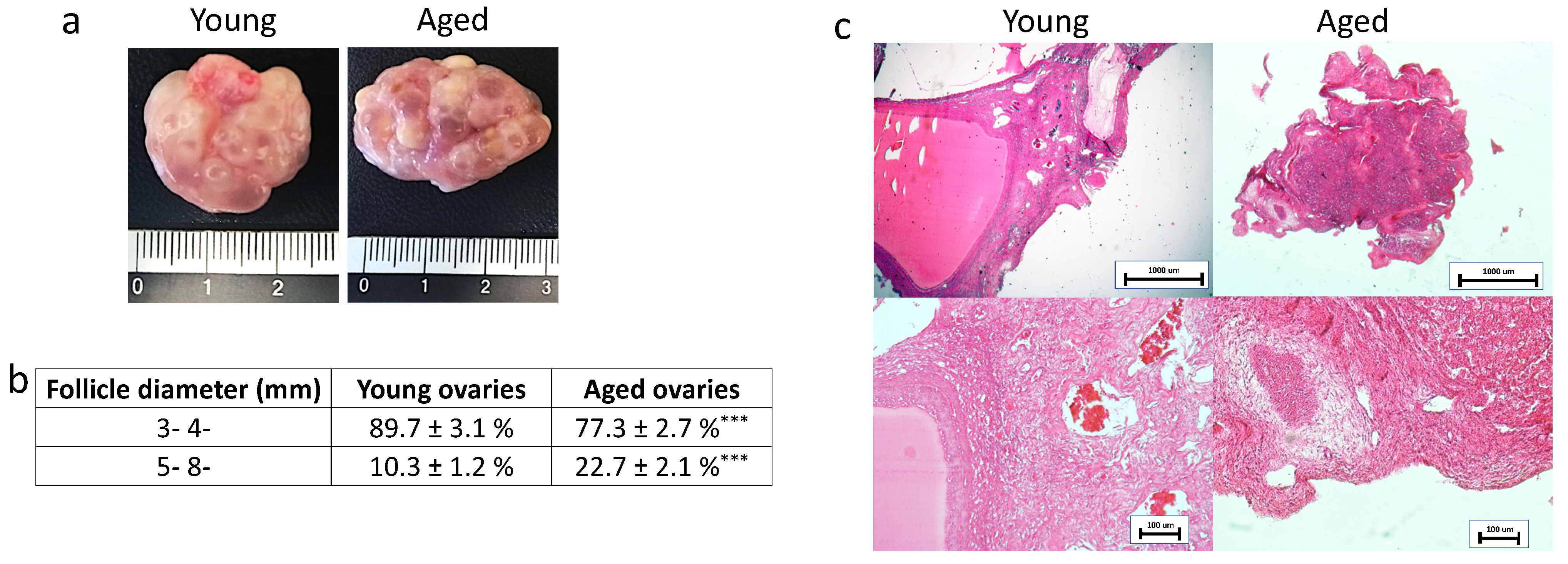

Figure 1. Macroscopic and histological evaluations of young and aged ovaries. (a) Representative macroscopic images illustrating young (left panel) and aged ovaries (right panels); (b) 3–4 mm and 5–8 mm follicle rate observed in young and aged ovaries, *** p < 0.001; (c) H & E staining showed the typical ovarian tissue architecture with primordial, growing and antral follicles, ovarian stroma and vasculature in both young and aged tissues. Scale bars = 100 μm.
In order to evaluate the general morphological aspect of young and aged ovaries, H&E staining was carried out. The analyzed tissues showed the typical ovarian tissue architecture consisting of primordial, growing, and antral follicles, ovarian stroma, and vasculature in both young and aged tissues. However, aged ovaries displayed a denser and more compact stromal compartment compared to the young ones (Figure 1c).
2.2. Aging Effects on Ovarian Extracellular Matrix Composition
In order to characterize the major ECM components, the researchers selected specific histological- and immuno-staining. In addition, a quantitative evaluation was carried out through stereological analysis and ELISA tests.
More in detail, Masson trichrome showed a robust blue staining in aged ovaries, indicating an increment in collagen content (Figure 2a). This was also confirmed by stereological analysis (Figure 2b) as well as ELISA quantifications (Figure 2c) that demonstrated significantly higher concentrations of collagen fibers in aged ovaries compared to their young counterpart.
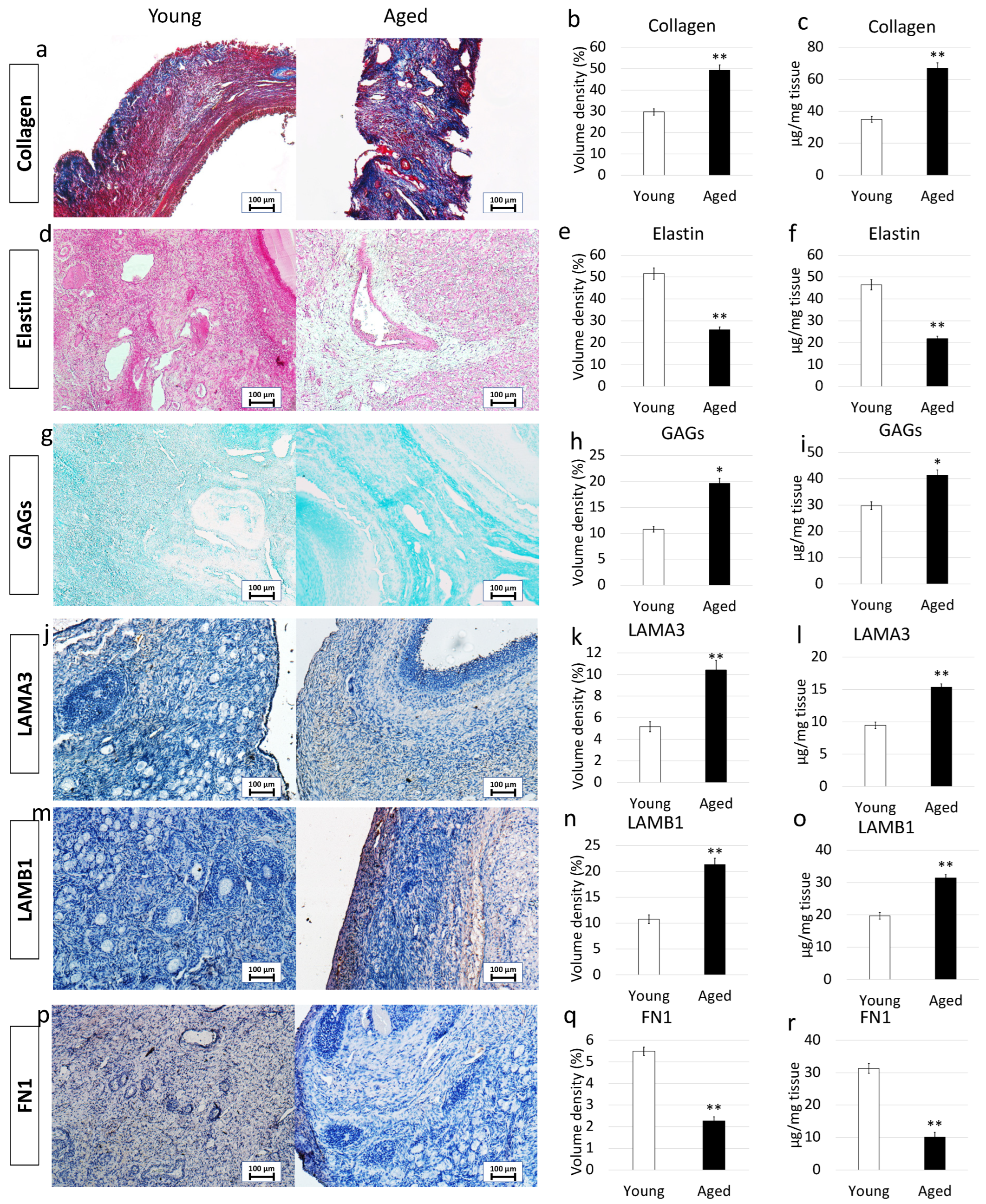

Figure 2. Histochemical and immunohistochemical analysis, stereological quantification, and ELISA tests in young and aged ovaries. (a) Masson’s trichrome staining showed the presence of collagen (blue) and elastic (magenta) fibers in both young and aged ovaries. Scale bars = 100 μm; (b) Stereological analysis demonstrated a significant increase of collagen fibers in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (c) ELISA quantifications indicated a significant increase of the collagen content in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (d) Gomori’s Aldehyde Fuchsin staining revealed the presence of elastic fibers in both young and aged ovaries. Scale bars = 100 μm; (e) Stereological analysis displayed a significant decrease of elastic fibers in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (f) ELISA quantifications confirmed a significant decrease of the elastin content in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (g) Alcian blue staining showed the presence of GAGs in both young and aged ovaries. Scale bars = 100 μm; (h) Stereological analysis indicated a significant increase of GAGs in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), * p < 0.05; (i) ELISA quantifications confirmed a significant increase of the GAG content in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), * p < 0.05; (j) Immunohistochemical staining of young and aged ovaries for LAMA3. Scale bars = 100 μm; (k) Stereological analysis demonstrated a significant increase of LAMA3 in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (l) ELISA quantifications showed a significant increase of the LAMA3 content in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (m) Immunohistochemical staining of young and aged ovaries for LAMB1. Scale bars = 100 μm; (n) Stereological analysis revealed a significant increase of LAMB1 in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (o) ELISA quantifications displayed a significant increase of the LAMB1 content in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (p) Immunohistochemical staining of young and aged ovaries for FN1. Scale bars = 100 μm; (q) Stereological analysis indicated a significant decrease in FN1 content in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (r) ELISA quantifications showed a significant increase of the LAMB1 content in aged ovaries compared to the young ones. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01.
Elastin specific staining Gomori’s Aldehyde Fuchsin demonstrated a decrease in elastic fiber content in aged samples (Figure 2d). This is further supported by stereological analysis (Figure 2e) and ELISA quantifications (Figure 2f), which indicated a significant decrease in elastic fiber volume density in aged ovaries.
In contrast, Alcian blu staining (Figure 2g) and stereological analysis (Figure 2h) demonstrated an increased quantity of GAGs in aged ovaries compared to the young ones. This was also confirmed by ELISA tests that indicated a significantly higher concentration of GAG content in aged ovaries (Figure 2i).
Immunohistochemical characterization demonstrated a higher amount of LAMA3 and LAMB1 in aged ovaries compared to the young group (Figure 2j,m). This was confirmed by stereological (Figure 2k,n) and ELISA quantifications (Figure 2l,o). On the other hand, FN1 staining (Figure 2p), stereological analysis (Figure 2q) and ELISA tests (Figure 2r) indicated a decreased fibronectin quantity in aged ovaries.
2.3. Aging Effects on Extracellular Matrix-Related Gene Expression
To confirm the morphological data revealing ECM composition changes with aging progression, the researchers profiled expression patterns and levels of ECM-related genes (Figure 3). While no significant modifications in expression patterns were detected, the researchers could assess a very dynamic response in the transcription levels of the genes considered. In particular, the researchers were able to demonstrate significantly increased expression levels of collagens (COL1A1, COL3A1, COL4A2), EMILIN1 glycoprotein, laminins (LAMA3, LAMB1), and proteoglycans (VCAN, HSPG2, CSPG4) in aged ovaries compared to the young ones. In contrast, down regulation of elastin (ELN)and fibronectin (FN1) accompanied the aging process which also caused a decreased expression of various proteases that hydrolyze the different ECM components, namely ELANE, MMP1, MMP2, MMP3, MMP9, and MMP14. Interestingly, the matrix metalloproteinase involved in elastin degradation, the MMP12, was upregulated in aged ovaries.
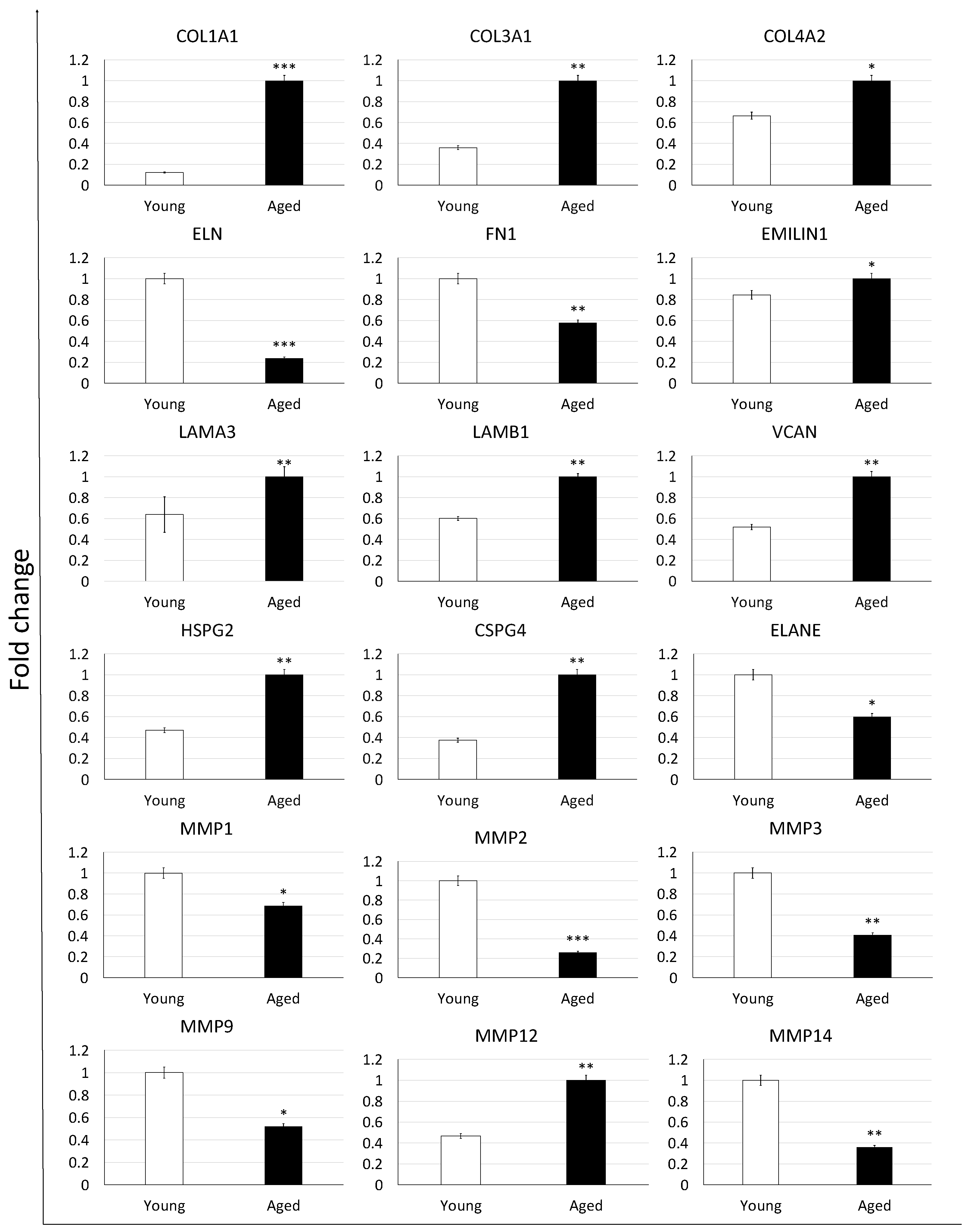

Figure 3. Gene expression changes of extracellular matrix-related genes in young (white bars) and aged ovaries (black bars). Expression levels of collagens (COL1A1, COL3A1, COL4A2), elastin (ELN), fibronectin (FN1), glycoprotein (EMILIN1), laminins (LAMA3, LAMB1), proteoglycans (VCAN, HSPG2, CSPG4), and proteases (ELANE, MMP1, MMP2, MMP3, MMP9, MMP12, and MMP14). Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), * p < 0.05, ** p < 0.01, *** p < 0.001.
2.4. Whole-Ovary Decellularization Protocol Successfully Removes Cell Compartment and Preserves Age-Specific Ovarian Architecture
In order to assess the efficacy of the whole-organ decellularization protocol, macroscopic evaluations were carried out along the decellularization process. The results obtained demonstrated that both young and aged ovaries preserved their original shape and homogeneity, without any deformation and maintained the age-related morphological differences already detectable in the native organs. In addition, comparable changes in whole organ color, turning from red to white, were visible in both the experimental groups (Figure 4a).
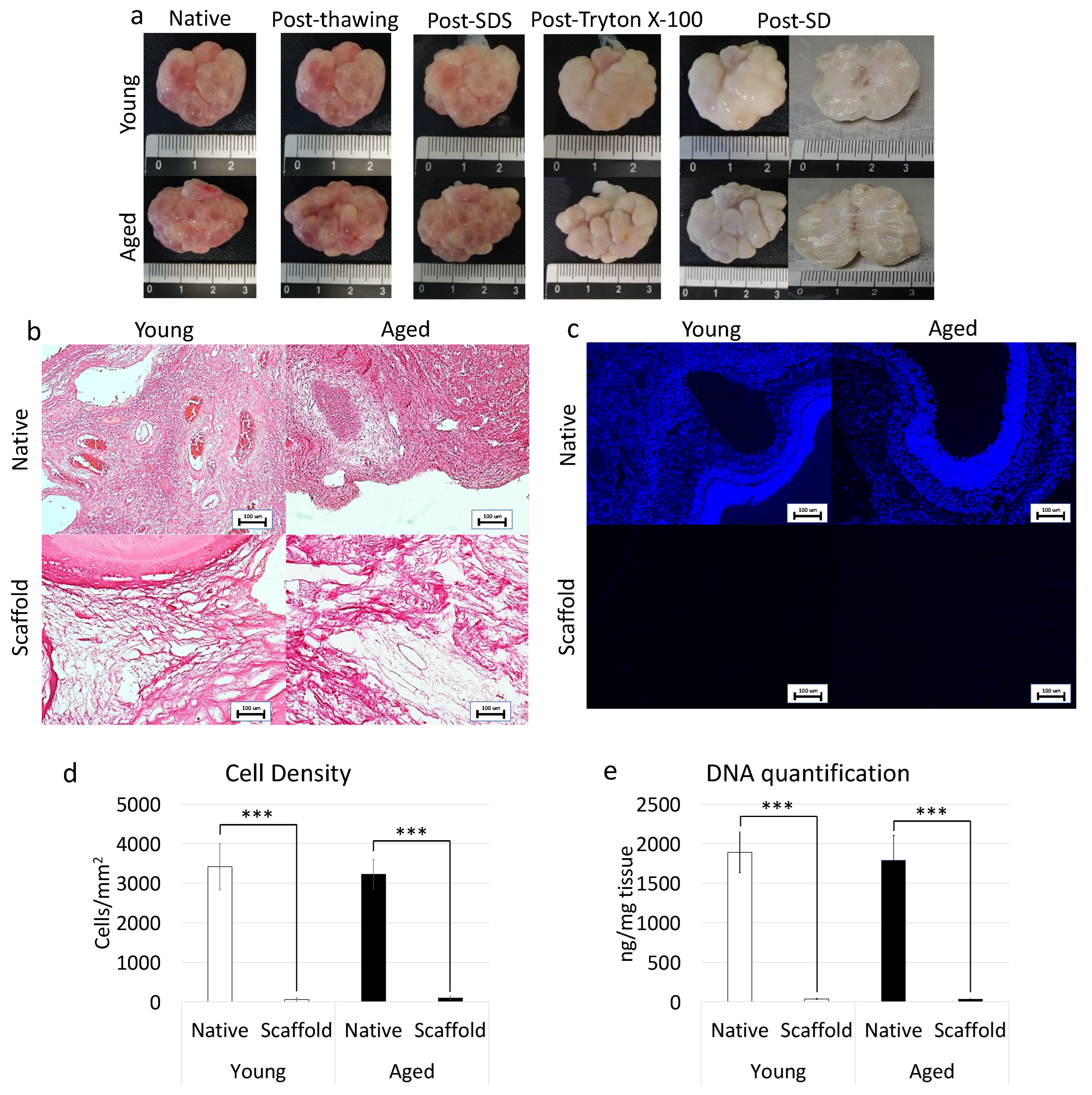

Figure 4. Macroscopic and microscopic evaluations of young and aged ECM-based scaffolds, cell density, and DNA quantification. (a) Chronological macroscopic images illustrating the decellularization process in young (upper panel) and aged ovaries (lower panels). Native and decellularized organs displayed comparable shapes and homogeneity, while their color turns from red to white along the process; (b) H&E staining showed the presence of both basophilic (cell nuclei) and eosinophilic (cell cytoplasm and ECM) staining in the control tissues (Young Native and Aged Native), while cell nuclei and the related basophilic staining were absent in the decellularized ECM-based scaffolds (Young Scaffold and Aged Scaffold). Scale bars = 100 μm; (c) DAPI staining displayed the presence of nuclei in native ovaries (Young Native and Aged Native), which disappeared after the decellularization process (Young Scaffold and Aged Scaffold). Scale bars = 100 μm; (d) Cell density demonstrated a significantly lower number of nuclei in both young and aged decellularized ECM-based scaffolds (Scaffold) compared to the untreated tissues (Native). Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), *** p < 0.001; (e) DNA quantification analysis showed a significant decrease in the DNA content of young and aged decellularized ECM-based scaffolds (Scaffold) compared to the native tissue (Native). Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), *** p < 0.001.
In addition, H&E staining was performed to evaluate the general morphological aspect of the decellularized whole-ovaries. The data obtained demonstrated the successful removal of the cellular compartment and the maintenance of general structure in both young and aged decellularized ovaries. More in detail, the basophilic staining, clearly visible in the native tissues, was absent after the decellularization processes in all samples analyzed, regardless of age (Figure 4b). In agreement with this, DAPI staining (Figure 4c) and cell density evaluations (Figure 4d) showed a significantly lower number of cell nuclei in both young and aged decellularized ovaries compared to the untreated tissues. This is also confirmed by DNA quantification studies indicating a 98.07% and a 98.22% decrease in young and aged decellularized ovaries, respectively. In particular, a content of 36.36 ± 4.54 ng DNA/mg and 31.75 ± 2.29 ng DNA/mg of tissue was measured in young and aged decellularized tissues vs. 1890.28 ± 257.37 ng DNA/mg and 1789.96 ± 313.55 ng DNA/mg of tissue in young and aged native controls (Figure 4e).
2.5. Whole-Ovary Decellularization Protocol Maintains Unaltered Aged-Specific Extracellular Matrix Composition
2.5.1. Histochemical Characterization
Maintenance of age-specific ECM composition and organization after decellularization was investigated using specific histochemical staining for collagen, elastin, and GAGs.
More in detail, Masson trichrome staining showed the persistence of collagen fibers in both young and aged decellularized ovaries, with comparable distribution between decellularized and native tissues belonging to the same age. In agreement with this, at the end of the decellularization processes, collagen fibers maintained the age-specific organization detected in the native tissues (Figure 5a). These morphological observations were also confirmed by stereological analysis, where no significant differences were detected between native and decellularized tissues of the same age (Figure 5b). In agreement with this, the age-related differences originally identified in the native tissues were maintained after the decellularization process (Figure 5b). These data were further supported by ELISA tests indicating comparable collagen content in young native and decellularized ovaries (35.1 ± 4.1 µg/mg and 32.3 ± 3.9 µg/mg of tissue) as well as in aged native and decellularized tissues (67.1 ± 9.1 µg/mg and 63.2 ± 8.9 µg/mg of tissue; Figure 5c).
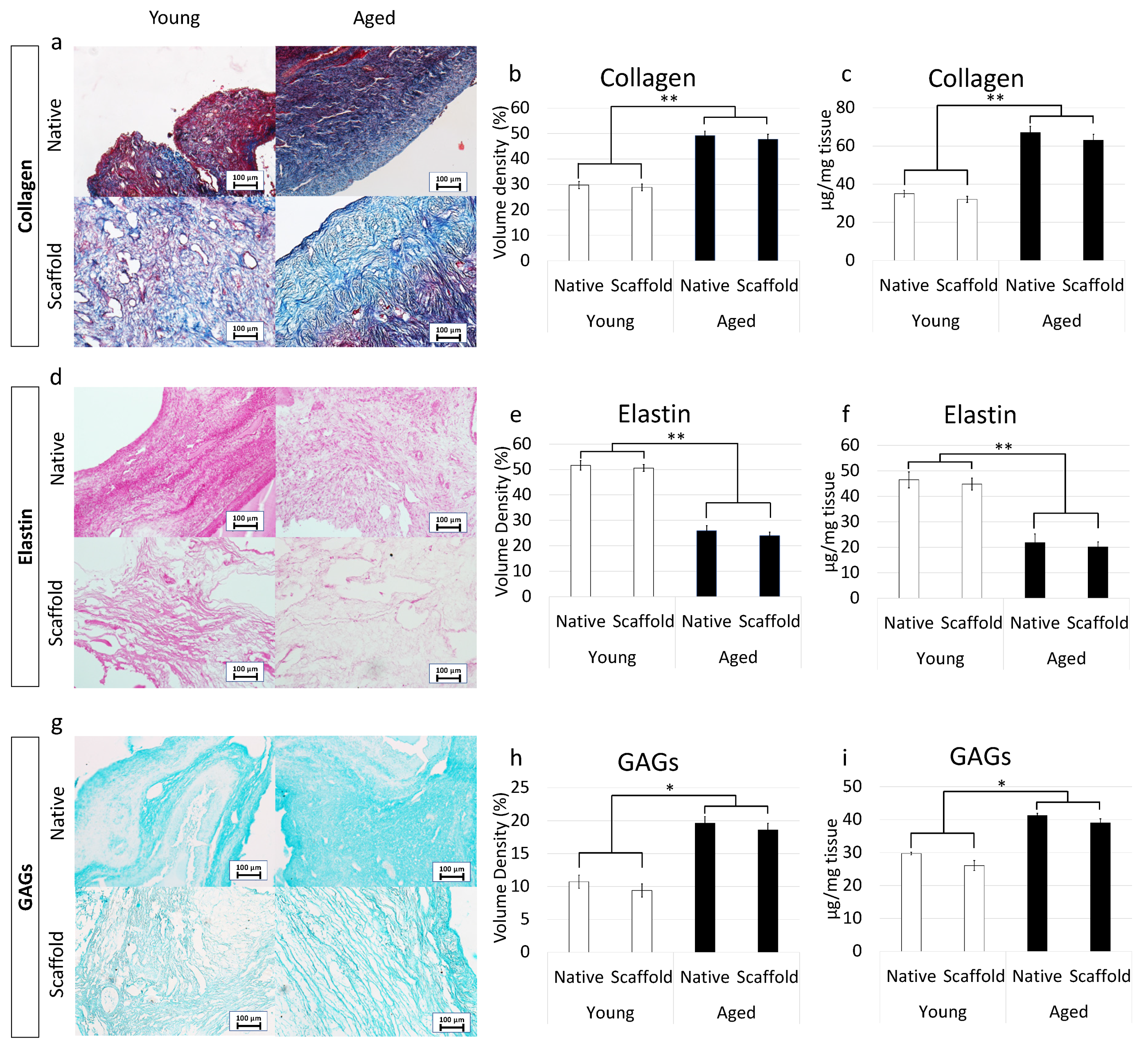

Figure 5. ECM microarchitecture and composition in young and aged ECM-based scaffolds. (a) Masson’s Trichrome staining showed the persistence of collagen fibers (blue) and their comparable distribution between native (Native) and decellularized ovaries (Scaffold) belonging to the same age. (b,c) Stereological (b) and ELISA quantifications (c) demonstrated no significant differences between native ovaries (Native) and the decellularized ECM-based scaffolds (Scaffold) in collagen content. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (d) Gomori’s aldehyde-fuchsin staining indicated that ECM-based scaffolds (Scaffold) retained the elastic fibers scattered throughout the decellularized ovary, similar to what was visible in untreated young and aged ovaries (Native). (e,f) Stereological (e) and ELISA quantifications (f) confirmed no significant differences between native ovaries (Native) and the decellularized ECM-based scaffolds (Scaffold) in elastin content. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (g) Alcian blue staining revealed GAG retention in young and aged decellularized scaffolds (Scaffold). Scale bars = 100 μm; (h,i) Stereological (h) and ELISA quantifications (i) demonstrated no significant differences between native ovaries (Native) and the decellularized ECM-based scaffolds (Scaffold) in GAG content. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), * p < 0.05.
Gomori’s aldehyde-fuchsin staining demonstrated that decellularized tissues preserved elastic fibers scattered throughout the ovary, regardless of age. Consistently, decellularized young and aged ovaries displayed the same differences observed in the native organs (Figure 5d). This was further supported by stereological analysis displaying no significant differences between young decellularized and native tissue as well as between aged decellularized and native ovaries (Figure 5e). Similarly, elastin quantification studies showed a comparable amount of elastin before and after the decellularization process in young (46.5 ± 3.12 µg/mg and 44.85 ± 2.32 µg/mg) and aged (21.87 ± 3.34 µg/mg and 20.09 ± 2.01 µg/mg) tissues (Figure 5f).
Alcian blue staining revealed GAG retention in both young and aged tissues, preserving the age-specific distributions visible in the native ovaries (Figure 5g). These morphological observations were confirmed by both stereological analysis (Figure 5h) and ELISA quantification studies (Figure 5i), which, as seen for the other ECM components, displayed no significant GAG reduction after the decellularization.
2.5.2. Immunohistochemical Characterization
Preservation of LAMA3, LAMB1, and FN1 in the produced bio-scaffold was investigated using immunohistochemical analysis. The results obtained demonstrated that decellularized ovaries maintained unaltered distribution of LAMA3, LAMB1, and FN1 compared to the untreated native tissues (Figure 6a,d,g). This was confirmed by stereological analysis indicating significantly lower volume densities of LAMA3 (Figure 6b) and LAMB1 (Figure 6e) in both native and decellularized young tissues compared to their aged counterparts. In contrast, FN1 content was higher in young samples as displayed by immunohistochemical staining (Figure 6g) and stereological analysis (Figure 6h). These evaluations were also supported by ELISA quantifications demonstrating a higher amount of LAMA3 (Figure 6c) and LAMB1 (Figure 6f) in aged native and decellularized tissues and lower content of FN1 (Figure 6i).
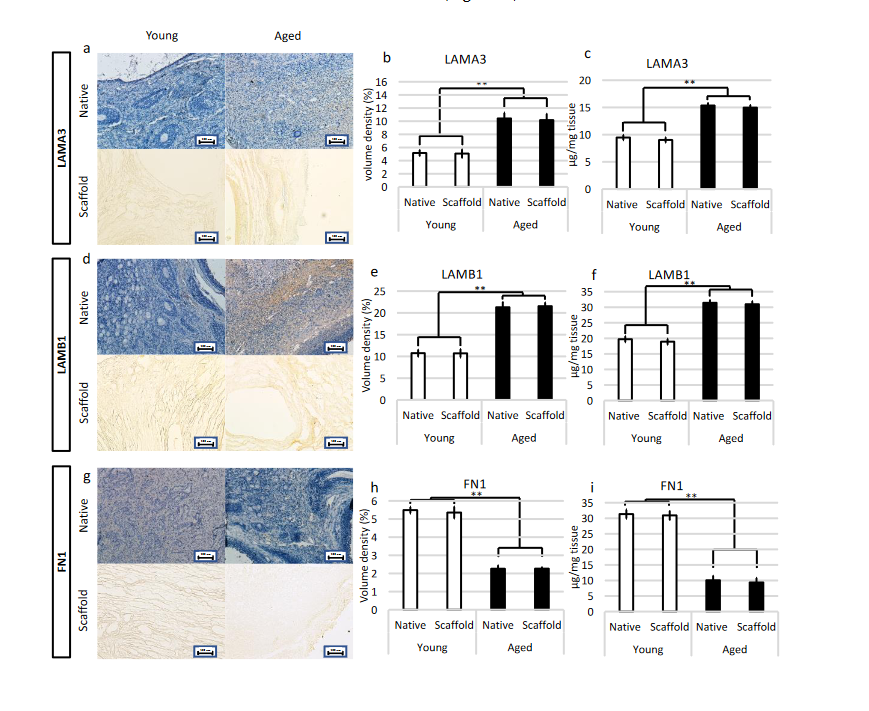

Figure 6. Immunohistochemical characterizations of young and aged ECM-based scaffolds. (a) LAMA3 immunostaining displayed the persistence of the fibers after decellularization in both young and aged tissues; Stereological analysis (b) and ELISA quantifications (c) demonstrated no significant differences between native (Native) and decellularized (Scaffold) ovaries in LAMA3 content. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (d) LAMB1 immunostaining showed the maintenance of the fibers at the end of the process in both young and aged organs; Stereological analysis (e) and ELISA tests (f) indicated no significant differences between native (Native) and decellularized tissues (Scaffold) in LAMB1 content. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01; (g) FN1 immunostaining revealed fiber retainment in both young and aged decellularized tissues; Stereological (h) and ELISA analysis (i) showed no significant differences between native (Native) and decellularized samples (Scaffold) in FN1 content. Data are expressed as the mean. Error bars represent the standard error of the mean (SEM), ** p < 0.01.
References
- Amargant, F.; Manuel, S.L.; Tu, Q.; Parkes, W.S.; Rivas, F.; Zhou, L.T.; Rowley, J.E.; Villanueva, C.E.; Hornick, J.E.; Shekhawat, G.S.; et al. Ovarian Stiffness Increases with Age in the Mammalian Ovary and Depends on Collagen and Hyaluronan Matrices. Aging Cell 2020, 19, e12359.
- Trinh, X.-B.; Peeters, F.; Tjalma, W.A.A. The Thoughts of Breast Cancer Survivors Regarding the Need for Starting Hormone Replacement Therapy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 124, 250–253.
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian Aging: Mechanisms and Clinical Consequences. Endocr. Rev. 2009, 30, 465–493.
- Gold, E.B. The Timing of the Age at Which Natural Menopause Occurs. Obstet. Gynecol. Clin. North Am. 2011, 38, 425–440.
- Li, C.J.; Lin, L.T.; Tsai, H.W.; Chern, C.U.; Wen, Z.H.; Wang, P.H.; Tsui, K.H. The Molecular Regulation in the Pathophysiology in Ovarian Aging. Aging Dis. 2021, 12, 934.
- Phillip, J.M.; Aifuwa, I.; Walston, J.; Wirtz, D. The Mechanobiology of Aging. Annu. Rev. Biomed. Eng. 2015, 17, 113.
- Briley, S.M.; Jasti, S.; McCracken, J.M.; Hornick, J.E.; Fegley, B.; Pritchard, M.T.; Duncan, F.E. Reproductive Age-Associated Fibrosis in the Stroma of the Mammalian Ovary. Reproduction 2016, 152, 245–260.
- Brevini, T.A.L.; Pennarossa, G.; Gandolfi, F. A 3D Approach to Reproduction. Theriogenology 2020, 150, 2–7.
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677.
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction Gone Awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73.
- Mammoto, A.; Ingber, D.E. Cytoskeletal Control of Growth and Cell Fate Switching. Curr. Opin. Cell Biol. 2009, 21, 864–870.
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in Development: A Growing Role for Contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43.
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195.
- Biernacka, A.; Frangogiannis, N.G. Aging and Cardiac Fibrosis. Aging Dis. 2011, 2, 158.
- Giménez, A.; Duch, P.; Puig, M.; Gabasa, M.; Xaubet, A.; Alcaraz, J. Dysregulated Collagen Homeostasis by Matrix Stiffening and TGF-Β1 in Fibroblasts from Idiopathic Pulmonary Fibrosis Patients: Role of FAK/Akt. Int. J. Mol. Sci. 2017, 18, 2431.
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K. The Role of Extracellular Matrix in Biomechanics and Its Impact on Bioengineering of Cells and 3D Tissues. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 85–86, 1–14.
- Thorne, J.T.; Segal, T.R.; Chang, S.; Jorge, S.; Segars, J.H.; Leppert, P.C. Dynamic Reciprocity between Cells and Their Microenvironment in Reproduction. Biol. Reprod. 2015, 92, 1–10.
- Woodruff, T.K.; Shea, L.D. A New Hypothesis Regarding Ovarian Follicle Development: Ovarian Rigidity as a Regulator of Selection and Health. J. Assist. Reprod. Genet. 2011, 28, 3–6.
- Hsueh, A.J.W.; Kawamura, K.; Cheng, Y.; Fauser, B.C.J.M. Intraovarian Control of Early Folliculogenesis. Endocr. Rev. 2015, 36, 1.
- Ouni, E.; Bouzin, C.; Dolmans, M.M.; Marbaix, E.; Pyr Dit Ruys, S.; Vertommen, D.; Amorim, C.A. Spatiotemporal Changes in Mechanical Matrisome Components of the Human Ovary from Prepuberty to Menopause. Hum. Reprod. 2020, 35, 1391–1410.
- Wood, C.D.; Vijayvergia, M.; Miller, F.H.; Carroll, T.; Fasanati, C.; Shea, L.D.; Catherine Brinson, L.; Woodruff, T.K. Multi-Modal Magnetic Resonance Elastography for Noninvasive Assessment of Ovarian Tissue Rigidity in Vivo. Acta Biomater. 2015, 13, 295–300.
- Hirshfeld-Cytron, J.E.; Duncan, F.E.; Xu, M.; Jozefik, J.K.; Shea, L.D.; Woodruff, T.K. Animal Age, Weight and Estrus Cycle Stage Impact the Quality of in Vitro Grown Follicles. Hum. Reprod. 2011, 26, 2473–2485.
More
