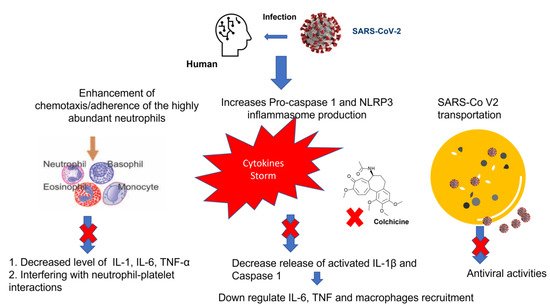
Despite the advance in the management of Coronavirus disease 2019 (COVID-19), the global pandemic is still ongoing with a massive health crisis. COVID-19 manifestations may range from mild symptoms to severe life threatening ones. The hallmark of the disease severity is related to the overproduction of pro-inflammatory cytokines manifested as a cytokine storm. Based on its anti-inflammatory activity through interfering with several pro and anti-inflammatory pathways, colchicine had been proposed to reduce the cytokine storm and subsequently improve clinical outcomes.
- COVID-19
- cytokine storm
- colchicine
- protease inhibitor
- molecular docking
- RNA polymerase
- SARS-CoV-2
1. Introduction
2. Colchicine Mechanism of Action
3. Molecular Docking Analysis of Colchicine against RNA-dependent RNA Polymerase and Protease Enzymes of SARS-CoV-2
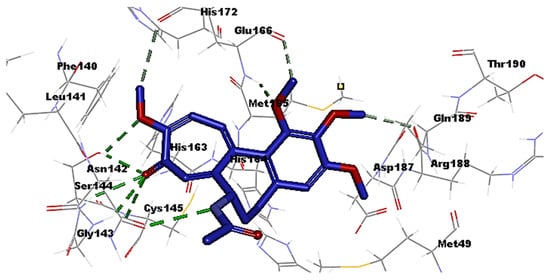
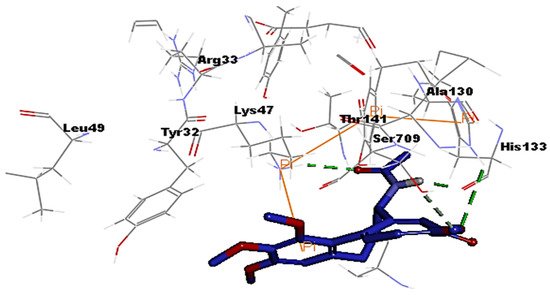
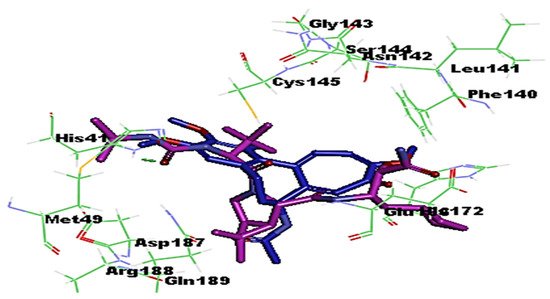
- Colchicine Pharmacokinetics
The mean oral bioavailability of colchicine is about 45% and, interestingly, the peripheral leukocytes remain its main compartment to accumulate [32]. In comparison to its concentration in plasma, colchicine potential to selectively accumulate in neutrophiles is about 16 times higher. This could be attributed to a deficiency in the expression of an efflux protein known as P-glycoprotein (P-gp) among neutrophils [33]. Colchicine is metabolized in the liver and intestine by cytochrome P450 3A4 (CYP3A4) and is eliminated into the bile and urine via P-gp. Accordingly, it is contraindicated to prescribe colchicine among patients with hepatic or renal deterioration and especially if they are receiving CYP3A4 or P-gp inhibitors [32].
At prescribed doses, colchicine is well tolerated despite of its narrow therapeutic index. The most common side effects are gastrointestinal ones that can occur in more than 20% of patients in addition to other rarer side effects, such as neuromyopathy that likely occurs with chronic daily use of colchicine [16]. Lopes et al. had reported on the effectiveness of colchicine maximum daily dose when taken as 0.5 mg three times daily for 5 days and then the same dose twice daily for another 5 days on reducing the length of hospital stay and need for supplemental oxygen therapy by a median of 7 and 4 days, respectively [34].
4.1. Rationale for using Colchicine in Controlling ARDS and MOD
The pulmonary manifestations of SARS-COV-2 are broad and mainly range from viral pneumonia to fatal ARDS (15% of cases) that is characterized by sudden respiratory failure due to over activation of the host immune response [35]. Hyper-inflammatory immune response the so-called “cytokine storm” will eventually correlate with COVID-19 severity, leading to exacerbation of respiratory failure and MOD due to extensive tissue damage. Of note, the synthesis of IL-6 induces the production of CRP, which is considered to be one of the first biomarkers associated with cytokine storm among COVID-19 patients [38]. In theory, colchicine expresses a wide array of anti-inflammatory activity that mainly targets the cytokine storm at different levels and additionally, as a significantly cheaper drug compared to the currently used antiviral agents, such as remdesivir or molnupiravir, with a great availability, it has lead us to explore its effect in improving and controlling ARDS and other extra pulmonary complications of COVID-19 [38].
In light of its anti-inflammatory activity, randomized clinical trials as the GRECCO-19 (NCT04326790) and the COLCORONA multicenter study (NCT04322682) had evaluated the effectiveness of colchicine in reducing inflammatory markers and thereby improving clinical outcomes [38]. The results were promising as a former study had shown a statistically significant improvement in clinical status [39], while the latter study had shown a lowered rate of mortality and hospitalization [40]. Indeed, another case control study had shown a statistically significant reduction of ferritin, CRP, and D-dimer with a p value of 0.012, 0.014, and 0.037, respectively [37]. So far, there are approximately 26 ongoing clinical studies on the effect of colchicine on improving the clinical status of COVID-19 patients [41]. Recently, a meta-analysis study that was conducted using several databases published from December 2019 until August 26, 2021 had reported on the efficacy of colchicine in reducing inflammatory biomarkers among moderate-to-severe patient cases of Covid-19. Of interest, they recommended conducting further clinical trials to ensure the effectiveness of colchicine as an adjuvant treatment for patients with COVID-19 [42].
On the other hand, a randomized control study conducted on fifty-six hospitalized patients, allocated to colchicine and sixty patients receiving placebo had reported on the ineffectiveness of colchicine in the treatment of severe COVID-19 despite being a safe drug [43]. Additionally, a meta-analysis of randomized controlled trials conducted by Mehta et al. proved a moderate quality evidence suggesting no benefit of addition of colchicine to the standard care regimen in patients with COVID-19 [44]. Another study, conducted between November 27, 2020 and March 4, 2021 showed that colchicine was not associated with reductions in mortality, duration of hospital stay, or risk of clinical complications [45]. Another study concluded that colchicine treatment neither improved the clinical status, nor the inflammatory response, over the standard treatment and advised that a preventive effect of colchicine on further clinical deterioration should be considered [46].
Accordingly, and based on these findings, much more well-designed multicenter studies are still required to direct clinicians upon the optimum use and duration of colchicine within hospitalized or non-hospitalized patients with COVID-19. In the context of the above rationale (immunomodulatory action and promising in silico antiviral activity), we hope to design the following multicenter clinical trial to evaluate the effectiveness of using colchicine as a prompt treatment for COVID-19. The inclusion criteria and patient settings are: Hospitalized COVID-19 patients (age ranging from 17–70) with a rising titer in biomarker profile namely IL1, IL-6, and TNFα. Rising titer could be determined by at least two discrete time-point measurement in any of the inflammatory markers.
Intervention: This study will depend on random distribution of participants into 3 groups (at 1:1:1 ratio).
Group 1: patient receiving maximum safety dose of colchicine alone.
Group 2: patient receiving maximum safety dose of colchicine in addition to standard treatment (combination therapy).
Group 3: patient receiving standard treatment alone (control group).
Primary outcomes:
Among the primary outcomes, one is to estimate the risk of clinical deterioration mainly through the need of mechanical ventilator or presence of cardiac arrhythmias. A second one is to estimate the length of hospital stay and to evaluate the risk of 30 day mortality rate. The third outcome is to report side effects associated with colchicine at the prescribed dose.
4.2. Potential Role of Colchicine in Suppressing Various Inflammatory Signaling Pathways and Their Associations with COVID-19
Mitogen-activated protein kinases (MAPK) are formerly known as extracellular signal-regulated kinases (ERK) and are sets of proteins located on the cell surface that mediate signal activation up on their binding to signal proteins to the nucleic acid of the cell [42]. The resulted signals activate the level of cellular protein expression producing certain cellular changes such as cell multiplication, proliferation, as well as inflammation, particularly during the COVID-19 infection [47–49]. Various studies have been reported on the potential role of colchicine for suppressing the MAPK signaling pathways and therefore, as a promising therapy to control the inflammation and clinically relevant complications associated with the activation of this pathway [50–52].
It was recently reported that neopterin (NPT), a protein synthesized by macrophages up on their activation by interferon gamma (INF-γ) has a potential role in mediating inflammation and various clinical complications associated with COVID-19 [53]. On the other hand, it was also reported that NPT has anti-inflammatory and antioxidant effects by down-regulating the expression of nuclear factor kappa B (NF-κB) signaling and NLRP3 inflammasomes and therefore overcome hyper-inflammation, oxidative stress, and accompanying organ failure [53]. Another study conducted by Itano et al. showed that colchicine was able to significantly inhibit the angiotensin II‑induced fibroblast migration in vitro and attenuated the renal fibrosis in a murine unilateral ureteral obstruction model [54]. It was also reported that colchicine in combination with nicorandil was able to prevent monocrotaline-induced rat pulmonary arterial hypertension [55].
Signal transducers and activators of transcription (STATs) are a group of proteins that are important for cancer survival, proliferation, metastases, and angiogenesis [56]. A study conducted in 2020 by Tantawy et al. confirmed the IL6/JAK2/STAT3 axis down-regulation as a major contributor for inhibiting the progression of lung carcinoma via influencing the cell apoptosis [57]. Various reports have documented the potential role of colchicine in the suppression of this inflammatory cascade pathway and therefore predicting its potential use with lung cancer and COVID-19 cytokines storm [57,58]. Autophagy is a natural, mechanism of the cell to eliminate unnecessary or dysfunctional cellular components via a lysosome-dependent regulated manner [59]. Colchicine was recently reported to interfere with the autophagy mechanism and therefore enhance the efficiency of cell transfection by DNA, conferring its potential activities against cancer, the virally infected cells, and other clinically relevant pathological conditions [60–62].
Furthermore, many recent studies have reported the use of colchicine for protecting against acute myocardial infarction, cardiomyopathy [63], and other clinically relevant conditions in humans such as renal ischemia, liver damage, and osteoarthritis [64,65] by modulating the macrophage polarization and suppressing the TLR4/NFκB/NLRP3 signal pathway and therefore, inhibiting pyroptosis and inflammatory response [63–65]. Recently, it was also reported that the cluster of differentiation 147 (CD147) transmembrane protein is a novel route for SARS-CoV-2 entry and therefore influences viral pathogenesis in humans [66]. A recent study conducted by Avolio et al. revealed that the SARS-CoV-2 Spike protein disrupts human cardiac pericytes’ function through CD147-receptor-mediated signaling [67]. However, the potential role of colchicine in suppressing such mechanism has not yet been confirmed.
4.3. Colchicine Dosage, Timing, and Drug–Drug Interaction in COVID-19 Positive Patients
Colchicine is a well-tolerated drug and the most common side effects are gastrointestinal, including diarrhea and colitis that can occur in more than 20% of patients in addition to other rarer side effects such as neuromyopathy that likely occurs with chronic daily use of colchicine [16]. It was previously reported that the colchicine maximum daily dose when taken as 0.5 mg three times daily for 5 days and then same dose twice daily for another 5 days reduces clinical complications by a median of 7 and 4 days, respectively [34]. Recently, Vitiello et al. reported that Low-dose colchicine could be considered safe and effective for controlling of the cytokine storm in patients with Covid-19, especially as an adjunctive medication to other treatment options [68].
A meta-analysis study on colchicine’s safety profile during the pre-COVID-19 time revealed that colchicine is a well-tolerated drug and has a good safety profile, except for the occurrence of gastrointestinal disorder [69]. Similar findings were observed in other meta-analysis pooled models among patients with COVID-19 [44,70]. It was reported that the metabolism of Colchicine can be decreased when combined with Remdesivir [71,72]. It was also reported that a fatal interaction occurred upon the coadministration of colchicine with p-glycoprotein and cytochrome P450 inhibitors such as ketoconazole, lopinavir, nelfinavir, ritonavir cyclosporine, and macrolides such as erythromycin and clarithromycin [73].
- Conclusions
The clinical progression of COVID-19 towards a more severe scenario is linked to the production of a cytokine storm. Colchicine has a great potential to inhibit the cytokine storm through interfering with several pro/anti-inflammatory pathways and NLRP3 activation. Docking analysis provided strong evidence of antiviral activity comparable to that of a standard antiprotease drug, boceprevir, by inhibiting the protease enzyme of SARS-CoV-2, and therefore, could prevent its replication. Nevertheless, the use of colchicine in the treatment of Covid-19 patients remains questionable. Hence, comprehensive clinical trials are required to show the effectiveness of repurposing colchicine, the old and low-cost anti-inflammatory drug, as a prompt treatment for patients with COVID-19.
- WHO. COVID-19Dashboard. Geneva2020 [2/6/2021]. Available online: https://covid19.who.int/ (accessed on 20 November 2021).
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [CrossRef]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [CrossRef] [PubMed]
- Dasgeb, B.; Kornreich, D.; McGuinn, K.; Okon, L.; Brownell, I.; Sackett, D.L. Colchicine: An ancient drug with novel applications.Br. J. Dermatol. 2018, 178, 350–356. [CrossRef]
- Reyes, A.Z.; Hu, K.A.; Teperman, J.; Muskardin, T.L.W.; Tardif, J.C.; Shah, B.; Pillinger, M.H. Anti-inflammatory therapy forCOVID-19 infection: The case for colchicine. Ann. Rheum. Dis. 2021, 80, 550–557. [CrossRef] [PubMed]
- Nolasco, S.; Bellido, J.; Serna, M.; Carmona, B.; Soares, H.; Zabala, J.C. Colchicine Blocks Tubulin Heterodimer Recycling by Tubulin Cofactors TBCA, TBCB, and TBCE. Front. Cell Dev. Biol. 2021, 9, 656273. [CrossRef] [PubMed]
- Phelps, P. Appearance of chemotactic activity following intra-articular injection of monosodium urate crystals: Effect of colchicine.J. Lab. Clin. Med. 1970, 76, 622–631. [PubMed]
- Perricone, C.; Bartoloni, E.; Gerli, R. Colchicine, an anti-rheumatic agent, as a potential compound for the treatment of COVID-19.Reumatologia 2020, 58, 261–264. [CrossRef]
- Cronstein, B.N.; Molad, Y.; Reibman, J.; Balakhane, E.; Levin, R.I.;Weissmann, G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J. Clin. Investig. 1995, 96, 994–1002. [CrossRef] [PubMed]
- Schlesinger, N.; Firestein, B.L.; Brunetti, L. Colchicine in COVID-19: An Old Drug, New Use. Curr. Pharmacol. Rep. 2020, 6, 137–145. [CrossRef] [PubMed]
- Nuki, G. Colchicine: Its mechanism of action and efficacy in crystal-induced inflammation. Curr. Rheumatol. Rep. 2008, 10, 218–227. [CrossRef]
- Lisman, T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2008, 371, 567–576.[CrossRef]
- Imazio, M.; Nidorf, M. Colchicine and the heart. Eur. Heart J. 2021, 42, 2745–2760. [CrossRef]
- Weng, J.H.; Koch, P.D.; Luan, H.H.; Tu, H.C.; Shimada, K.; Ngan, I.; Ventura, R.; Jiang, R.; Mitchison, T.J. Colchicine acts selectivelyin the liver to induce hepatokines that inhibit myeloid cell activation. Nat. Metab. 2021, 3, 513–522. [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev.Immunol. 2019, 19, 477–489. [CrossRef]
- Slobodnick, A.; Shah, B.; Krasnokutsky, S.; Pillinger, M.H. Update on colchicine, 2017. Rheumatology 2017, 57, i4–i11. [CrossRef][PubMed]
- Naghavi, M.H.;Walsh, D. Microtubule Regulation and Function during Virus Infection. J. Virol. 2017, 91, e00538-17. [CrossRef]
- Kamel, N.A.; El Wakeel, L.M.; Aboshanab, K.M. Exploring SARS-CoV-2 Spikes Glycoproteins for Designing Potential Antiviral Targets. Viral Immunol. 2021, 34, 510–521. [CrossRef] [PubMed]
- Ren, L.; Zhang, Y.; Li, J.; Xiao, Y.; Zhang, J.; Wang, Y.; Chen, L.; Paranhos-Baccalà, G.; Wang, J. Genetic drift of human coronavirus OC43 spike gene during adaptive evolution. Sci. Rep. 2015, 5, 11451. [CrossRef] [PubMed]
- Wen, Z.; Zhang, Y.; Lin, Z.; Shi, K.; Jiu, Y. Cytoskeleton—a crucial key in host cell for coronavirus infection. J. Mol. Cell Biol. 2020,12, 968–979. [CrossRef]
- Cao, Y.; Yang, R.; Lee, I.; Zhang, W.; Sun, J.; Wang, W.; Meng, X. Characterization of the SARS-CoV-2 E Protein: Sequence,Structure, Viroporin, and Inhibitors. Protein Sci. 2021, 30, 1114–1130. [CrossRef]
- Chen, I.-Y.; Moriyama, M.; Chang, M.-F.; Ichinohe, T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front. Microbiol. 2019, 10, 50. [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [CrossRef]
- Rastogi, M.; Pandey, N.; Shukla, A.; Singh, S.K. SARS coronavirus 2: From genome to infectome. Respir. Res. 2020, 21, 318.[CrossRef] [PubMed]
- Mody, V.; Ho, J.; Wills, S.; Mawri, A.; Lawson, L.; Ebert, M.C.C.J.C.; Fortin, G.M.; Rayalam, S.; Taval, S. Identification of3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 2020, 20, 93. [CrossRef]
- Aftab, S.O.; Ghouri, M.Z.; Masood, M.U.; Haider, Z.; Khan, Z.; Ahmad, A.; Munawar, N. Analysis of SARS-CoV-2 RNA-dependentRNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020, 18, 275. [CrossRef][PubMed]
- Rameshkumar, M.R.; Indu, P.; Arunagirinathan, N.; Venkatadri, B.; El-Serehy, H.A.; Ahmad, A. Computational selection offlavonoid compounds as inhibitors against SARS-CoV-2 main protease, RNA-dependent RNA polymerase and spike proteins: Amolecular docking study. Saudi J. Biol. Sci. 2021, 28, 448–458. [CrossRef]
- Abo-zeida, Y.; Ismail, N.S.M.; McLean, G.R.; Hamdy, N.M. A molecular docking study repurposes FDA approved iron oxidenanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [CrossRef]
- Kneller, D.W.; Galanie, S.; Phillips, G.; O’Neill, H.M.; Coates, L.; Kovalevsky, A. Malleability of the SARS-CoV-2 3CL M proActive-Site Cavity Facilitates Binding of Clinical Antivirals. Structure 2020, 28, 1313. [CrossRef]Medicina 2022, 58, 20 10 of 11
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P.Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 279. [CrossRef]
- Parvez, M.S.; Karim, M.A.; Hasan, M.; Jaman, J.; Karim, Z.; Tahsin, T.; Hasan, M.N.; Hosen, M.J. Prediction of potential inhibitorsfor RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach.Int. J. Biol. Macromol. 2020, 163, 1787–1797. [CrossRef] [PubMed]
- Karatza, E.; Ismailos, G.; Karalis, V. Colchicine for the treatment of COVID-19 patients: Efficacy, safety, and model informed dosage regimens. Xenobiotica 2021, 51, 643–656. [CrossRef]
- Leung, Y.Y.; Hui, L.L.Y.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum.2015, 45, 341–350. [CrossRef]
- Lopes, M.I.; Bonjorno, L.P.; Giannini, M.C.; Amaral, N.B.; Menezes, P.I.; Dib, S.M.; Gigante, S.L.; Benatti, M.N.; Rezek, U.C.;Emrich-Filho, L.L.; et al. Beneficial effects of colchicine for moderate to severe COVID-19: A randomised, double-blinded,placebo-controlled clinical trial. RMD Open 2021, 7, e001455. [CrossRef] [PubMed]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [CrossRef] [PubMed]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; WhatWe Know So Far. Front. Immunol.2020, 11, 1446. [CrossRef] [PubMed]
- Sandhu, T.; Tieng, A.; Chilimuri, S.; Franchin, G. A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 8865954. [CrossRef]
- Manenti, L.; Maggiore, U.; Fiaccadori, E.; Meschi, T.; Antoni, A.D.; Nouvenne, A.; Ticinesi, A.; Cerundolo, N.; Prati, B.;Delsante, M.; et al. Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observationalstudy. PLoS ONE 2021, 16, e0248276. [CrossRef]
- Deftereos, S.G.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.D.; Giotaki, S.G.; Gargalianos, P.; Metallidis, S.; Sianos, G.;Baltagiannis, S.; Panagopoulos, P.; et al. Effect of Colchicine vs. Standard Care on Cardiac and Inflammatory Biomarkers andClinical Outcomes in Patients Hospitalized with Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2013136. [CrossRef]
- Tardif, J.C.; Bouabdallaoui, N.; L’Allier, P.L.; Gaudet, D.; Shah, B.; Pillinger, M.H.; Lopez-Sendon, J.; da Luz, P.; Verret, L.; Audet, S.; et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): A phase 3, randomised, doubleblinded,adaptive, placebo-controlled, multicentre trial. Lancet Respir. Med. 2021, 9, 924–932. [CrossRef]
- Scientific Advisory Group. COVID-19 Scientific Advisory Group Rapid Evidence Report. 2021. Available online: https://www.albertahealthservices.ca/topics/Page17074.aspx (accessed on 20 November 2021).
- Sarwar, M.; Ali, Z.; Fatima, M.; Sarfraz, Z.; Sarfraz, A.; Cherrez-Ojeda, I. Colchicine, COVID-19 and hematological parameters: A meta-analysis. J. Clin. Lab. Anal. 2021, 35, e24057. [CrossRef]
- Absalón-Aguilar,A.; Rull-Gabayet,M.; Pérez-Fragoso,A.;Mejía-Domínguez,N.R.;Núñez-Álvarez, C.; Kershenobich-Stalnikowitz, D.; Sifuentes-Osornio, J.; Ponce-de-León, A.; González-Lara, F.; Martín-Nares, E.; et al. Colchicine Is Safe Though Ineffective in theTreatment of Severe COVID-19: A Randomized Clinical Trial (COLCHIVID). J. Gen. Intern. Med. 2021, 9, 1–11. [CrossRef]
- Mehta, K.G.; Patel, T.; Chavda, P.D.; Patel, P. Efficacy and safety of colchicine in COVID-19: A meta-analysis of randomized controlled trials. RMD Open 2021, 7, e001746. [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet Respir. Med. 2021, 9, 1419–1426. [CrossRef]
- Pascual-Figal, D.A.; Roura-Piloto, A.E.; Moral-Escudero, E.; Bernal, E.; Albendín-Iglesias, H. Pérez-Martínez, M.T.; Noguera-Velasco, J.A.; Cebreiros-López, I.; Hernández-Vicente, Á.; Vázquez-Andrés, D. et al. Colchicine in Recently Hospitalized Patients with COVID-19: A Randomized Controlled Trial (COL-COVID). Int. J. Gen. Med. 2021, 14, 5517–5526.[CrossRef]
- Orton, R.J.; Sturm, O.E.; Vyshemirsky, V.; Calder, M.; Gilbert, D.R.; Kolch, W. Computational modelling of the receptor-tyrosinekinase-activated MAPK pathway. Biochem. J. 2005, 392, 249–261. [CrossRef] [PubMed]
- Raghavan, S.; Kundumani-Sridharan, V.; Kumar, S.; White, C.W.; Das, K.C. Thioredoxin Prevents Loss of UCP2 in Hyperoxia via MKK4-p38 MAPK- PGC1_ Signaling and Limits Oxygen Toxicity. Am. J. Respir. Cell Mol. Biol. 2021. ahead of print. [CrossRef]
- Shahgolzari, M.; Yavari, A.; Arjeini, Y.; Miri, S.M.; Darabi, A.; Mozaffari Nejad, A.S.; Keshavarz, M. Immunopathology andImmunopathogenesis of COVID-19, what we know and what we should learn. Gene Rep. 2021, 25, 101417. [CrossRef] [PubMed]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz-Górzy ´ nska, M.; Ławi´ nski, J.; Rysz, J. Emerging Anti-Atherosclerotic Therapies. Int. J. Mol.Sci. 2021, 22, 2109. [CrossRef]
- Voutyritsa, E.; Kyriakos, G.; Patsouras, A.; Damaskos, C.; Garmpi, A.; Diamantis, E.; Garmpis, N.; Savvanis, S. ExperimentalAgents for the Treatment of Atherosclerosis: New Directions. J. Exp. Pharmacol. 2021, 13, 161–179. [CrossRef]
- Liu, Z.; Wang, C.; Wang, Y.; Wang, L.; Zhang, Y.; Yan, G. 4’-O-Methylbroussochalcone B as a novel tubulin polymerizationinhibitor suppressed the proliferation and migration of acute myeloid leukaemia cells. BMC Cancer 2021, 21, 91. [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alzahrani, K.J.; Cruz-Martins, N.; Batiha, G.E. The potential role of neopterin in COVID-19: A new perspective. Mol. Cell Biochem. 2021, 476, 4161–4166. [CrossRef]
- Itano, S.; Satoh, M.; Kadoya, H.; Sogawa, Y.; Uchida, A.; Sasaki, T.; Kashihara, N. Colchicine attenuates renal fibrosis in a murineunilateral ureteral obstruction model. Mol. Med. Rep. 2017, 15, 4169–4175. [CrossRef]Medicina 2022, 58, 20 11 of 11
- Lee, F.Y.; Lu, H.I.; Zhen, Y.Y.; Leu, S.; Chen, Y.L.; Tsai, T.H.; Chung, S.Y.; Chua, S.; Sheu, J.J.; Hsu, S.Y.; et al. Benefit of combinedtherapy with nicorandil and colchicine in preventing monocrotaline-induced rat pulmonary arterial hypertension. Eur. J. Pharm.Sci. 2013, 50, 372–384. [CrossRef]
- Aggarwal, B.B.; Kunnumakkara, A.B.; Harikumar, K.B.; Gupta, S.R.; Tharakan, S.T.; Koca, C.; Dey, S.; Sung, B. Signal transducerand activator of transcription-3, inflammation, and cancer: How intimate is the relationship? Ann. N. Y. Acad. Sci. 2009, 1171,59–76. [CrossRef] [PubMed]
- Tantawy, M.A.; Shaheen, S.; Kattan, S.W.; Alelwani,W.; Barnawi, I.O.; Elmgeed, G.A.; Nafie, M.S. Cytotoxicity, in silico predictionsand molecular studies for androstane heterocycle compounds revealed potential antitumor agent against lung cancer cells. J.Biomol. Struct. Dyn. 2020, 10, 1–14. [CrossRef] [PubMed]
- Talukdar, J.; Bhadra, B.; Dattaroy, T.; Nagle, V.; Dasgupta, S. Potential of natural astaxanthin in alleviating the risk of cytokinestorm in COVID-19. Biomed. Pharmacother. 2020, 132, 110886. [CrossRef]
- Klionsky, D.J. Autophagy revisited: A conversation with Christian de Duve. Autophagy 2008, 4, 740–743. [CrossRef] [PubMed]
- Tsuchiya, M.; Ogawa, H.; Watanabe, K.; Koujin, T.; Mori, C.; Nunomura, K.; Lin, B.; Tani, A.; Hiraoka, Y.; Haraguchi, T.Microtubule inhibitors identified through nonbiased screening enhance DNA transfection efficiency by delaying p62-dependent ubiquitin recruitment. Genes Cells. 2021, 26, 739–751. [CrossRef]
- Das Mukherjee, D.; Kumar, N.M.; Tantak, M.P.; Datta, S.; Ghosh Dastidar, D.; Kumar, D.; Chakrabarti, G. NMK-BH2. NMK-BH2, a novel microtubule-depolymerising bis (indolyl)-hydrazide-hydrazone, induces apoptotic and autophagic cell death in cervicalcancer cells by binding to tubulin at colchicine-site. Biochim. Biophys. Acta Mol. Cell. Res. 2020, 1867, 118762. [CrossRef]
- Buch, B.T.; Halling, J.F.; Ringholm, S.; Gudiksen, A.; Kjøbsted, R.; Olsen, M.A.; Wojtaszewski, J.F.P.; Pilegaard, H. Colchicine treatment impairs skeletal muscle mitochondrial function and insulin sensitivity in an age-specific manner. FASEB J. 2020, 34, 8653–8670. [CrossRef]
- Wang, L.; Peng, Y.; Song, L.; Xia, D.; Li, C.; Li, Z.; Li, Q.; Yu, A.; Lu, C.; Wang, Y. Colchicine-Containing Nanoparticles Attenuates Acute Myocardial Infarction Injury by Inhibiting Inflammation. Cardiovasc. Drugs Ther. [CrossRef] [PubMed]
- Awad, A.S.; Elariny, H.A.; Sallam, A.S. Colchicine attenuates renal ischemia-reperfusion-induced liver damage: Implication ofTLR4/NF-_B, TGF-_, and BAX and Bcl-2 gene expression. Can. J. Physiol. Pharmacol. 2021, 19, 1–7. [CrossRef] [PubMed]
- Elmazoglu, Z.; Bek, Z.A.; Sarıba¸s, S.G.; Özo˘ gul, C.; Goker, B.; Bitik, B.; Aktekin, C.N.; Karasu, Ç. S-allylcysteine inhibits chondrocyte inflammation to reduce human osteoarthritis via targeting RAGE, TLR4, JNK, and Nrf2 signaling: Comparison withcolchicine. Biochem. Cell Biol. 2021, 99, 645–654. [CrossRef]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interactionin COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2021, 808, 152072. [CrossRef]
- Avolio, E.; Carrabba, M.; Milligan, R.; Kavanagh Williamson, M. Beltrami, A.P.; Gupta, K.; Elvers, K.T.; Gamez, M.; Foster, R.R.; Gillespie, K. et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147-receptor-mediated signalling: A potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. 2021, 135, 2667–2689. [CrossRef]
- Vitiello, A.; Ferrara, F. Colchicine and SARS-CoV-2: Management of the hyperinflammatory state. Respir. Med. 2021, 178, 106322.[CrossRef]
- Stewart, S.; Yang, K.C.K.; Atkins, K.; Dalbeth, N.; Robinson, P.C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2020, 22, 28. [CrossRef]
- Mareev, V.Y.; Orlova, Y.A.; Plisyk, A.G.; Pavlikova, E.P.; Akopyan, Z.A.; Matskeplishvili, S.T.; Malakhov, P.S.; Krasnova, T.N.; Seredenina, E.M.; Potapenko, A.V.; et al. Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study. Kardiologiia 2021, 61, 15–27. [CrossRef]
- Yang, K. What DoWe Know About Remdesivir Drug Interactions? Clin. Transl. Sci. 2020, 13, 842–844. [CrossRef] [PubMed]
- FDA: Fact Sheet for Health Care Providers EUA of Remdesivir. Available online: https://www.fda.gov/media/137566/download (accessed on 20 November 2021).
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicinepoisoning: The dark side of an ancient drug. Clin. Toxicol. 2010, 48, 407–414. [CrossRef]
4. Colchicine Pharmacokinetics
4.1. Rationale for using Colchicine in Controlling ARDS and MOD
- Group 1:
-
patient receiving maximum safety dose of colchicine alone.
- Group 2:
-
patient receiving maximum safety dose of colchicine in addition to standard treatment (combination therapy).
- Group 3:
-
patient receiving standard treatment alone (control group).Primary outcomes:Among the primary outcomes, one is to estimate the risk of clinical deterioration mainly through the need of mechanical ventilator or presence of cardiac arrhythmias. A second one is to estimate the length of hospital stay and to evaluate the risk of 30 day mortality rate. The third outcome is to report side effects associated with colchicine at the prescribed dose.
4.2. Potential Role of Colchicine in Suppressing Various Inflammatory Signaling Pathways and Their Associations with COVID-19
Mitogen-activated protein kinases (MAPK) are formerly known as extracellular signal-regulated kinases (ERK) and are sets of proteins located on the cell surface that mediate signal activation up on their binding to signal proteins to the nucleic acid of the cell [42]. The resulted signals activate the level of cellular protein expression producing certain cellular changes such as cell multiplication, proliferation, as well as inflammation, particularly during the COVID-19 infection [47][48][49]. Various studies have been reported on the potential role of colchicine for suppressing the MAPK signaling pathways and therefore, as a promising therapy to control the inflammation and clinically relevant complications associated with the activation of this pathway [50][51][52].It was recently reported that neopterin (NPT), a protein synthesized by macrophages up on their activation by interferon gamma (INF-γ) has a potential role in mediating inflammation and various clinical complications associated with COVID-19 [53]. On the other hand, it was also reported that NPT has anti-inflammatory and antioxidant effects by down-regulating the expression of nuclear factor kappa B (NF-κB) signaling and NLRP3 inflammasomes and therefore overcome hyper-inflammation, oxidative stress, and accompanying organ failure [53]. Another study conducted by Itano et al. showed that colchicine was able to significantly inhibit the angiotensin II-induced fibroblast migration in vitro and attenuated the renal fibrosis in a murine unilateral ureteral obstruction model [54]. It was also reported that colchicine in combination with nicorandil was able to prevent monocrotaline-induced rat pulmonary arterial hypertension [55].Signal transducers and activators of transcription (STATs) are a group of proteins that are important for cancer survival, proliferation, metastases, and angiogenesis [56]. A study conducted in 2020 by Tantawy et al. confirmed the IL6/JAK2/STAT3 axis down-regulation as a major contributor for inhibiting the progression of lung carcinoma via influencing the cell apoptosis [57]. Various reports have documented the potential role of colchicine in the suppression of this inflammatory cascade pathway and therefore predicting its potential use with lung cancer and COVID-19 cytokines storm [57][58]. Autophagy is a natural, mechanism of the cell to eliminate unnecessary or dysfunctional cellular components via a lysosome-dependent regulated manner [59]. Colchicine was recently reported to interfere with the autophagy mechanism and therefore enhance the efficiency of cell transfection by DNA, conferring its potential activities against cancer, the virally infected cells, and other clinically relevant pathological conditions [60][61][62].Furthermore, many recent studies have reported the use of colchicine for protecting against acute myocardial infarction, cardiomyopathy [63], and other clinically relevant conditions in humans such as renal ischemia, liver damage, and osteoarthritis [64][65] by modulating the macrophage polarization and suppressing the TLR4/NFκB/NLRP3 signal pathway and therefore, inhibiting pyroptosis and inflammatory response [63][64][65]. Recently, it was also reported that the cluster of differentiation 147 (CD147) transmembrane protein is a novel route for SARS-CoV-2 entry and therefore influences viral pathogenesis in humans [66]. A recent study conducted by Avolio et al. revealed that the SARS-CoV-2 Spike protein disrupts human cardiac pericytes’ function through CD147-receptor-mediated signaling [67]. However, the potential role of colchicine in suppressing such mechanism has not yet been confirmed.4.3. Colchicine Dosage, Timing, and Drug–Drug Interaction in COVID-19 Positive Patients
Colchicine is a well-tolerated drug and the most common side effects are gastrointestinal, including diarrhea and colitis that can occur in more than 20% of patients in addition to other rarer side effects such as neuromyopathy that likely occurs with chronic daily use of colchicine [16]. It was previously reported that the colchicine maximum daily dose when taken as 0.5 mg three times daily for 5 days and then same dose twice daily for another 5 days reduces clinical complications by a median of 7 and 4 days, respectively [34]. Recently, Vitiello et al. reported that Low-dose colchicine could be considered safe and effective for controlling of the cytokine storm in patients with COVID-19, especially as an adjunctive medication to other treatment options [68].A meta-analysis study on colchicine’s safety profile during the pre-COVID-19 time revealed that colchicine is a well-tolerated drug and has a good safety profile, except for the occurrence of gastrointestinal disorder [69]. Similar findings were observed in other meta-analysis pooled models among patients with COVID-19 [44][70]. It was reported that the metabolism of Colchicine can be decreased when combined with Remdesivir [71][72]. It was also reported that a fatal interaction occurred upon the coadministration of colchicine with p-glycoprotein and cytochrome P450 inhibitors such as ketoconazole, lopinavir, nelfinavir, ritonavir cyclosporine, and macrolides such as erythromycin and clarithromycin [73].
References
- WHO. COVID-19 Dashboard. Geneva2020 . Available online: https://covid19.who.int/ (accessed on 20 November 2021).Karatza, E.; Ismailos, G.; Karalis, V. Colchicine for the treatment of COVID-19 patients: Efficacy, safety, and model informed dosage regimens. Xenobiotica 2021, 51, 643–656.
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. Leung, Y.Y.; Hui, L.L.Y.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350.
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. Slobodnick, A.; Shah, B.; Krasnokutsky, S.; Pillinger, M.H. Update on colchicine, 2017. Rheumatology 2017, 57, i4–i11.
- Dasgeb, B.; Kornreich, D.; McGuinn, K.; Okon, L.; Brownell, I.; Sackett, D.L. Colchicine: An ancient drug with novel applications. Br. J. Dermatol. 2018, 178, 350–356. Lopes, M.I.; Bonjorno, L.P.; Giannini, M.C.; Amaral, N.B.; Menezes, P.I.; Dib, S.M.; Gigante, S.L.; Benatti, M.N.; Rezek, U.C.; Emrich-Filho, L.L.; et al. Beneficial effects of colchicine for moderate to severe COVID-19: A randomised, double-blinded, placebo-controlled clinical trial. RMD Open 2021, 7, e001455.
- Reyes, A.Z.; Hu, K.A.; Teperman, J.; Muskardin, T.L.W.; Tardif, J.C.; Shah, B.; Pillinger, M.H. Anti-inflammatory therapy for COVID-19 infection: The case for colchicine. Ann. Rheum. Dis. 2021, 80, 550–557. Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37.
- Nolasco, S.; Bellido, J.; Serna, M.; Carmona, B.; Soares, H.; Zabala, J.C. Colchicine Blocks Tubulin Heterodimer Recycling by Tubulin Cofactors TBCA, TBCB, and TBCE. Front. Cell Dev. Biol. 2021, 9, 656273. Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446.
- Phelps, P. Appearance of chemotactic activity following intra-articular injection of monosodium urate crystals: Effect of colchicine. J. Lab. Clin. Med. 1970, 76, 622–631. Sandhu, T.; Tieng, A.; Chilimuri, S.; Franchin, G. A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 8865954.
- Perricone, C.; Bartoloni, E.; Gerli, R. Colchicine, an anti-rheumatic agent, as a potential compound for the treatment of COVID-19. Reumatologia 2020, 58, 261–264. Manenti, L.; Maggiore, U.; Fiaccadori, E.; Meschi, T.; Antoni, A.D.; Nouvenne, A.; Ticinesi, A.; Cerundolo, N.; Prati, B.; Delsante, M.; et al. Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study. PLoS ONE 2021, 16, e0248276.
- Cronstein, B.N.; Molad, Y.; Reibman, J.; Balakhane, E.; Levin, R.I.; Weissmann, G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J. Clin. Investig. 1995, 96, 994–1002. Deftereos, S.G.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.D.; Giotaki, S.G.; Gargalianos, P.; Metallidis, S.; Sianos, G.; Baltagiannis, S.; Panagopoulos, P.; et al. Effect of Colchicine vs. Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized with Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2013136.
- Schlesinger, N.; Firestein, B.L.; Brunetti, L. Colchicine in COVID-19: An Old Drug, New Use. Curr. Pharmacol. Rep. 2020, 6, 137–145. Tardif, J.C.; Bouabdallaoui, N.; L’Allier, P.L.; Gaudet, D.; Shah, B.; Pillinger, M.H.; Lopez-Sendon, J.; da Luz, P.; Verret, L.; Audet, S.; et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): A phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir. Med. 2021, 9, 924–932.
- Nuki, G. Colchicine: Its mechanism of action and efficacy in crystal-induced inflammation. Curr. Rheumatol. Rep. 2008, 10, 218–227. Scientific Advisory Group. COVID-19 Scientific Advisory Group Rapid Evidence Report. 2021. Available online: https://www.albertahealthservices.ca/topics/Page17074.aspx (accessed on 20 November 2021).
- Lisman, T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2008, 371, 567–576. Sarwar, M.; Ali, Z.; Fatima, M.; Sarfraz, Z.; Sarfraz, A.; Cherrez-Ojeda, I. Colchicine, COVID-19 and hematological parameters: A meta-analysis. J. Clin. Lab. Anal. 2021, 35, e24057.
- Imazio, M.; Nidorf, M. Colchicine and the heart. Eur. Heart J. 2021, 42, 2745–2760. Absalón-Aguilar, A.; Rull-Gabayet, M.; Pérez-Fragoso, A.; Mejía-Domínguez, N.R.; Núñez-Álvarez, C.; Kershenobich-Stalnikowitz, D.; Sifuentes-Osornio, J.; Ponce-de-León, A.; González-Lara, F.; Martín-Nares, E.; et al. Colchicine Is Safe Though Ineffective in the Treatment of Severe COVID-19: A Randomized Clinical Trial (COLCHIVID). J. Gen. Intern. Med. 2021, 9, 1–11.
- Weng, J.H.; Koch, P.D.; Luan, H.H.; Tu, H.C.; Shimada, K.; Ngan, I.; Ventura, R.; Jiang, R.; Mitchison, T.J. Colchicine acts selectively in the liver to induce hepatokines that inhibit myeloid cell activation. Nat. Metab. 2021, 3, 513–522. Mehta, K.G.; Patel, T.; Chavda, P.D.; Patel, P. Efficacy and safety of colchicine in COVID-19: A meta-analysis of randomised controlled trials. RMD Open 2021, 7, e001746.
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. RECOVERY Collaborative Group. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet Respir. Med. 2021, 9, 1419–1426.
- Slobodnick, A.; Shah, B.; Krasnokutsky, S.; Pillinger, M.H. Update on colchicine, 2017. Rheumatology 2017, 57, i4–i11. Pascual-Figal, D.A.; Roura-Piloto, A.E.; Moral-Escudero, E.; Bernal, E.; Albendín-Iglesias, H. Pérez-Martínez, M.T.; Noguera-Velasco, J.A.; Cebreiros-López, I.; Hernández-Vicente, Á.; Vázquez-Andrés, D. et al. Colchicine in Recently Hospitalized Patients with COVID-19: A Randomized Controlled Trial (COL-COVID). Int. J. Gen. Med. 2021, 14, 5517–5526.
- Naghavi, M.H.; Walsh, D. Microtubule Regulation and Function during Virus Infection. J. Virol. 2017, 91, e00538-17. Orton, R.J.; Sturm, O.E.; Vyshemirsky, V.; Calder, M.; Gilbert, D.R.; Kolch, W. Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem. J. 2005, 392, 249–261.
- Kamel, N.A.; El Wakeel, L.M.; Aboshanab, K.M. Exploring SARS-CoV-2 Spikes Glycoproteins for Designing Potential Antiviral Targets. Viral Immunol. 2021, 34, 510–521. Raghavan, S.; Kundumani-Sridharan, V.; Kumar, S.; White, C.W.; Das, K.C. Thioredoxin Prevents Loss of UCP2 in Hyperoxia via MKK4-p38 MAPK- PGC1α Signaling and Limits Oxygen Toxicity. Am. J. Respir. Cell Mol. Biol. 2021. ahead of print.
- Ren, L.; Zhang, Y.; Li, J.; Xiao, Y.; Zhang, J.; Wang, Y.; Chen, L.; Paranhos-Baccalà, G.; Wang, J. Genetic drift of human coronavirus OC43 spike gene during adaptive evolution. Sci. Rep. 2015, 5, 11451. Shahgolzari, M.; Yavari, A.; Arjeini, Y.; Miri, S.M.; Darabi, A.; Mozaffari Nejad, A.S.; Keshavarz, M. Immunopathology and Immunopathogenesis of COVID-19, what we know and what we should learn. Gene Rep. 2021, 25, 101417.
- Wen, Z.; Zhang, Y.; Lin, Z.; Shi, K.; Jiu, Y. Cytoskeleton—a crucial key in host cell for coronavirus infection. J. Mol. Cell Biol. 2020, 12, 968–979. Gluba-Brzózka, A.; Franczyk, B.; Rysz-Górzyńska, M.; Ławiński, J.; Rysz, J. Emerging Anti-Atherosclerotic Therapies. Int. J. Mol. Sci. 2021, 22, 2109.
- Cao, Y.; Yang, R.; Lee, I.; Zhang, W.; Sun, J.; Wang, W.; Meng, X. Characterization of the SARS-CoV-2 E Protein: Sequence, Structure, Viroporin, and Inhibitors. Protein Sci. 2021, 30, 1114–1130. Voutyritsa, E.; Kyriakos, G.; Patsouras, A.; Damaskos, C.; Garmpi, A.; Diamantis, E.; Garmpis, N.; Savvanis, S. Experimental Agents for the Treatment of Atherosclerosis: New Directions. J. Exp. Pharmacol. 2021, 13, 161–179.
- Chen, I.-Y.; Moriyama, M.; Chang, M.-F.; Ichinohe, T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front. Microbiol. 2019, 10, 50. Liu, Z.; Wang, C.; Wang, Y.; Wang, L.; Zhang, Y.; Yan, G. 4’-O-Methylbroussochalcone B as a novel tubulin polymerization inhibitor suppressed the proliferation and migration of acute myeloid leukaemia cells. BMC Cancer 2021, 21, 91.
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alzahrani, K.J.; Cruz-Martins, N.; Batiha, G.E. The potential role of neopterin in COVID-19: A new perspective. Mol. Cell Biochem. 2021, 476, 4161–4166.
- Rastogi, M.; Pandey, N.; Shukla, A.; Singh, S.K. SARS coronavirus 2: From genome to infectome. Respir. Res. 2020, 21, 318. Itano, S.; Satoh, M.; Kadoya, H.; Sogawa, Y.; Uchida, A.; Sasaki, T.; Kashihara, N. Colchicine attenuates renal fibrosis in a murine unilateral ureteral obstruction model. Mol. Med. Rep. 2017, 15, 4169–4175.
- Mody, V.; Ho, J.; Wills, S.; Mawri, A.; Lawson, L.; Ebert, M.C.C.J.C.; Fortin, G.M.; Rayalam, S.; Taval, S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 2020, 20, 93. Lee, F.Y.; Lu, H.I.; Zhen, Y.Y.; Leu, S.; Chen, Y.L.; Tsai, T.H.; Chung, S.Y.; Chua, S.; Sheu, J.J.; Hsu, S.Y.; et al. Benefit of combined therapy with nicorandil and colchicine in preventing monocrotaline-induced rat pulmonary arterial hypertension. Eur. J. Pharm. Sci. 2013, 50, 372–384.
- Aftab, S.O.; Ghouri, M.Z.; Masood, M.U.; Haider, Z.; Khan, Z.; Ahmad, A.; Munawar, N. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020, 18, 275. Aggarwal, B.B.; Kunnumakkara, A.B.; Harikumar, K.B.; Gupta, S.R.; Tharakan, S.T.; Koca, C.; Dey, S.; Sung, B. Signal transducer and activator of transcription-3, inflammation, and cancer: How intimate is the relationship? Ann. N. Y. Acad. Sci. 2009, 1171, 59–76.
- Rameshkumar, M.R.; Indu, P.; Arunagirinathan, N.; Venkatadri, B.; El-Serehy, H.A.; Ahmad, A. Computational selection of flavonoid compounds as inhibitors against SARS-CoV-2 main protease, RNA-dependent RNA polymerase and spike proteins: A molecular docking study. Saudi J. Biol. Sci. 2021, 28, 448–458. Tantawy, M.A.; Shaheen, S.; Kattan, S.W.; Alelwani, W.; Barnawi, I.O.; Elmgeed, G.A.; Nafie, M.S. Cytotoxicity, in silico predictions and molecular studies for androstane heterocycle compounds revealed potential antitumor agent against lung cancer cells. J. Biomol. Struct. Dyn. 2020, 10, 1–14.
- Abo-zeida, Y.; Ismail, N.S.M.; McLean, G.R.; Hamdy, N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020, 153, 105465. Talukdar, J.; Bhadra, B.; Dattaroy, T.; Nagle, V.; Dasgupta, S. Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19. Biomed. Pharmacother. 2020, 132, 110886.
- Kneller, D.W.; Galanie, S.; Phillips, G.; O’Neill, H.M.; Coates, L.; Kovalevsky, A. Malleability of the SARS-CoV-2 3CL M pro Active-Site Cavity Facilitates Binding of Clinical Antivirals. Structure 2020, 28, 1313. Klionsky, D.J. Autophagy revisited: A conversation with Christian de Duve. Autophagy 2008, 4, 740–743.
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 279. Tsuchiya, M.; Ogawa, H.; Watanabe, K.; Koujin, T.; Mori, C.; Nunomura, K.; Lin, B.; Tani, A.; Hiraoka, Y.; Haraguchi, T. Microtubule inhibitors identified through nonbiased screening enhance DNA transfection efficiency by delaying p62-dependent ubiquitin recruitment. Genes Cells. 2021, 26, 739–751.
- Parvez, M.S.; Karim, M.A.; Hasan, M.; Jaman, J.; Karim, Z.; Tahsin, T.; Hasan, M.N.; Hosen, M.J. Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach. Int. J. Biol. Macromol. 2020, 163, 1787–1797. Das Mukherjee, D.; Kumar, N.M.; Tantak, M.P.; Datta, S.; Ghosh Dastidar, D.; Kumar, D.; Chakrabarti, G. NMK-BH2. NMK-BH2, a novel microtubule-depolymerising bis (indolyl)-hydrazide-hydrazone, induces apoptotic and autophagic cell death in cervical cancer cells by binding to tubulin at colchicine-site. Biochim. Biophys. Acta Mol. Cell. Res. 2020, 1867, 118762.
- Buch, B.T.; Halling, J.F.; Ringholm, S.; Gudiksen, A.; Kjøbsted, R.; Olsen, M.A.; Wojtaszewski, J.F.P.; Pilegaard, H. Colchicine treatment impairs skeletal muscle mitochondrial function and insulin sensitivity in an age-specific manner. FASEB J. 2020, 34, 8653–8670.
- Wang, L.; Peng, Y.; Song, L.; Xia, D.; Li, C.; Li, Z.; Li, Q.; Yu, A.; Lu, C.; Wang, Y. Colchicine-Containing Nanoparticles Attenuates Acute Myocardial Infarction Injury by Inhibiting Inflammation. Cardiovasc. Drugs Ther.
- Awad, A.S.; Elariny, H.A.; Sallam, A.S. Colchicine attenuates renal ischemia-reperfusion-induced liver damage: Implication of TLR4/NF-κB, TGF-β, and BAX and Bcl-2 gene expression. Can. J. Physiol. Pharmacol. 2021, 19, 1–7.
- Elmazoglu, Z.; Bek, Z.A.; Sarıbaş, S.G.; Özoğul, C.; Goker, B.; Bitik, B.; Aktekin, C.N.; Karasu, Ç. S-allylcysteine inhibits chondrocyte inflammation to reduce human osteoarthritis via targeting RAGE, TLR4, JNK, and Nrf2 signaling: Comparison with colchicine. Biochem. Cell Biol. 2021, 99, 645–654.
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2021, 808, 152072.
- Avolio, E.; Carrabba, M.; Milligan, R.; Kavanagh Williamson, M. Beltrami, A.P.; Gupta, K.; Elvers, K.T.; Gamez, M.; Foster, R.R.; Gillespie, K. et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147-receptor-mediated signalling: A potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. 2021, 135, 2667–2689.
- Vitiello, A.; Ferrara, F. Colchicine and SARS-CoV-2: Management of the hyperinflammatory state. Respir. Med. 2021, 178, 106322.
- Stewart, S.; Yang, K.C.K.; Atkins, K.; Dalbeth, N.; Robinson, P.C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2020, 22, 28.
- Mareev, V.Y.; Orlova, Y.A.; Plisyk, A.G.; Pavlikova, E.P.; Akopyan, Z.A.; Matskeplishvili, S.T.; Malakhov, P.S.; Krasnova, T.N.; Seredenina, E.M.; Potapenko, A.V.; et al. Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study. Kardiologiia 2021, 61, 15–27.
- Yang, K. What Do We Know About Remdesivir Drug Interactions? Clin. Transl. Sci. 2020, 13, 842–844.
- FDA: Fact Sheet for Health Care Providers EUA of Remdesivir. Available online: https://www.fda.gov/media/137566/download (accessed on 20 November 2021).
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicine poisoning: The dark side of an ancient drug. Clin. Toxicol. 2010, 48, 407–414.
