The combination of molecular imprinting technology with magnetic nanoparticles provides a new class of smart hybrids, i.e., magnetic molecularly imprinted polymers (MMIPs) to overcome limitations in current cancer therapy. The application of these complexes is gaining more interest in therapy, due to their favorable properties, namely, the ability to be guided and to generate slight hyperthermia with an appropriate external magnetic field, alongside the high selectivity and loading capacity of imprinted polymers toward a template molecule. In cancer therapy, using the MMIPs as smart-drug-delivery robots can be a promising alternative to conventional direct administered chemotherapy, aiming to enhance drug accumulation/penetration into the tumors while fewer side effects on the other organs.
- molecular imprinting technology
- magnetic molecularly imprinted polymers
- magnetic nanoparticles
- chemotherapy
- cancer
- smart-drug-delivery system
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Molecularly Imprinted Technology toward Drug-Delivery System (DDS)
Molecularly imprinted technology (MIT) is a step further into the design of polymeric NPs. This technology has become an established strategy, but it is still considered a burgeoning method toward biomedical applications. MIT allows for producing smart materials in nano and larger sizes with active sites that match the target compound’s size and functionality, the so-called template, within a polymeric matrix. Generally, the copolymerization of a liquid mixture containing porogenic solvent(s), functional monomers, template molecules, and crosslinkers with a careful design leads to the development of molecularly imprinted polymers (MIPs). The responsibility of creating intermolecular interactions with the template molecule is by functional monomers through either covalent or non-covalent bonds, whereas crosslinkers form the polymer scaffold around the template [1][35]. The obtained MIPs possess tailored cavities resembling the original template in terms of size, shape, and orientation [2][36]. Regarding drug delivery, due to the intermolecular interactions like hydrogen bonds, dipole-dipole, and ionic interactions between the template molecule and polymer functional groups, these cavities are capable of enhancing the NPs loading capacity, improving drug stability, solubility and adjusting the drug release kinetics [3][4][5][4,37,38]. An intelligent or smart drug release is the anticipated release of a therapeutic agent on-demand. For this aim, these MIPs can react to the external stimulations, making changes in their structure or the strength of interactions between the polymer functional groups and the template captured in the cavities. This feature is highly suitable for DDS as it allows the drugs to be released only upon a particular change in the environment (Figure 1) [1] [35] such as heat, pH changes, light, electric or magnetic fields, enzymes, reduction, and ultrasound waves [6][7][8,29]. The combination of stimuli-sensitivity and imprinting technology potentially leads to a high loading capacity of the template by imprinting, while the response to the external stimuli modulates the affinity of the polymeric network for the template molecule, providing the regulatory or switching capability of the loading/release processes [4][37].
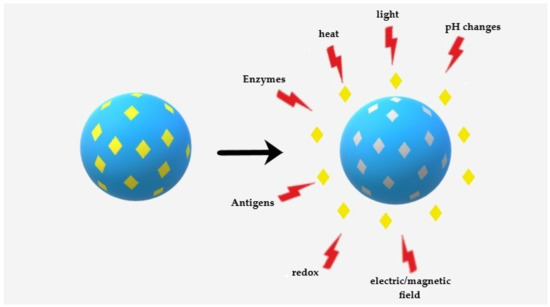
Figure 1.
The main advantages of MIPs in comparison with biomolecules such as antibodies and biologic receptors are their relatively high stability over various conditions and low cost [4][37]. MIPs have stable spatial structure and long-lasting shelf life that can be up to several years at room temperature [5] [38] and exceptional physical robustness and stability against tough conditions, including highly acidic and basic pH, temperature fluctuation, organic solvents and mechanical and thermal pressures [2][8][9][36,39,40]. In addition, compared to the non-imprinted polymeric NPs, the chief advantages of MIPs are their high selectivity and affinity for the target molecule, leading to the higher loading capacity and potential lower dose-dumping and immature burst release of the cargo [5][38]. They have been implemented in the development of biosensors [10][41], antibody mimics [11][42], catalysis [12][43], molecular recognition [13][44], drug delivery [14][15][45,46], diagnostics [16][47] and other biomedical applications [2][36]. As drug-delivery carriers, they have favorable specific binding tendency and loading, stability under different harsh settings, flexibility, and antibody-like recognition [17][48].
Noticeable progress has been gained in this area and many nanoMIP-based DDSs with largely improved sustained-release drug-delivery ability compared to their control polymers have been developed for different kinds of drugs intended to be used in various diseases [18][49]. The constraints concerning MIPs still need to be addressed, such as slow binding kinetics, aqueous compatibility, permeability in order to the drug extraction, and heterogeneity of binding site distributions [6][4][8,37]. However, there is a high chance for anticancer drugs to be transported by these carriers easily cross the cytoplasmic and nuclear membrane that will bring them with the in situ delivery with intact concentration and consequently, higher efficiency in the elimination of the tumor cell than that administered alone by conventional chemotherapy [19][50].
Anticancer drugs such as doxorubicin, 5-fluorouracil, and paclitaxel were utilized as a template of MIPs often to achieve controlled/sustained release of these drugs as well as better bioavailability, protection of the drug from fast degradation, diminish the adverse effects and efficient localized effect for potential chemotherapy of various cancers [20][21][22][23][24][51,52,53,54,55]. Bai et al. reported high drug loading (17.1%) and encapsulation efficiency (85.5%), as well as the desirable pH-dependent release (much faster release at pH 5 than those at pH 7) with a very slow and controlled release of paclitaxel imprinted system [20][51]. Similar outcomes were also reported by other groups [21][23][52,54].
The concept of MIT has a long history back to the early 1930 s. However, the preparation of organic polymers with molecular recognition as we know it today was first reported only in 1972 when two independent laboratories of Wulff and Klotz reported the preparation of organic polymers with a preselected ligand. Template molecules that were present during polymerization or derivatives were recognized better by the resultant structures [4][25][26][37,56,57]. Later on, a magnetically assisted DDS (MADDS) was introduced by Widder and coworkers in 1978, applying inorganic magnetic material in the structure of MIPs [27][58]. The combination of a magnetic core covered by a thin MIP shell leads to the generation of smart hybrid structures, namely magnetic MIPs (MMIPs) that provides the possibility of high drug loading and low off-target drug release followed by remote guidance, rapid distribution, and local accumulation of the obtained MMIPs by using an external magnetic field [28][59]. Due to the good biocompatibility and chemical, thermal and mechanical stability, high sorption capacity, high selectivity, reusability, low cost, and facile preparation method of the available magnetic materials, especially magnetic NPs (MNPs), the design of MMIPs as DDSs is recently become favorable [29][30][60,61]. Due to the high surface-to-volume-ratio of MNP, compared to MIPs, the imprinting position of the polymer at the surface is increased leading to MMIPs with more accessible imprinted positions, rapid mass transfer and hence, fewer permeability issues, as well as strong anti-interference ability [31][62].
One of the main obstacles toward nanocarriers’ efficacy in drug delivery could be the lack of knowledge about the precise bio-distribution, location, and subsequent therapeutic effects, as most studies have not examined the targeting efficiency of NPs real-time in vivo [32][28]. With this regard, among the active targeted MIP-based systems [22][23][33][34][35][36][53,54,63,64,65,66], MMIPs are appealing [22][34][35][53,64,65] because of the ease of active remote guidance to the site of interest in the body by using an external magnetic field. Regarding tumor chemotherapy, this feature largely enhances the drug concentration in the tumor tissues by much lower costs and potentially improves its therapeutic efficacy while narrowing the adverse toxicity to healthy cells through tumor local accumulation [18][49].
2. Magnetic Molecularly Imprinted Polymers, Promising Hybrid Nano-DDS
Besides ligand-mediated targeting, physical targeting can be achieved by adding some specific physical properties to the DDSs. One of the most interesting features could be the magnetic force which can accumulate magnetic materials in the specific region, in this content, tumor location, by use of a magnetic field [37][22].
Nowadays, MNPs, find vast applications in medicine, analytical chemistry, and biotechnology. The most commonly used MNPs includes metal or metal oxide NPs [38][67]. Iron oxide-based MNPs (Fe2O3, Fe3O4) especially the only clinically approved MNP, superparamagnetic iron oxide NP (SPION) are extensively investigated in nanomedicine for their biocompatibility, stability, eco-friendliness, low toxicity, contrast agent properties, ability to generate heat when submitted to an alternating magnetic field (hyperthermia) and intrinsic magnetic properties, i.e., superparamagnetism that allows them to exhibit magnetic properties only in the presence of an applied magnetic field [39][40][9,68]. Considering the property of superparamagnetism, they are broadly investigated in different clinical applications [38][67], especially as imaging agents. Feridex®/Endorem® and GastroMARK™; Umirem® (AMAG pharmaceuticals) are SPION NPs coated with dextran and silicone, respectively that due to their superparamagnetic character, were approved as imaging agents [41][21].
Magnetic drug targeting (MDT) involves enriching SPIONs at the area of interest via a strong external magnetic field and, consequently, potentially enables more specific and efficient treatment. MDT of drug-loaded SPIONs is indeed closer to application in patients [37][22]. Successful employment of SPIONs for cancer treatment was demonstrated by the complete tumor remission without significant side effects followed by the administration of mitoxantrone−SPIONs (with only 5–10% of the conventional chemotherapeutic dose) through the tumor-supplying vessel in rabbits and the application of a strong external magnetic field over the tumor location. The distribution profile after MDT displayed 66.3% of the particles localized in the tumor region with magnetic targeting, compared to less than 1% of drug and NPs reaching the tumor region during conventional intravenous application [42][69].
Furthermore, applying a thin imprinted polymer shell on the surface of the MNPs leads to enhancement in physicochemical properties for the intended MDT by enhancing binding kinetic, high surface-to-volume-ratio, increasing binding capacity, uniform spherical shape, and also monodispersity in aqueous blood circulatory [31][62]. Due to the presence of MNPs in their structure, they can induce the so-called magnetic hyperthermia (local heat enhancement) when submitted to an external magnetic field (subsequent release of the drug when MMIP is loaded). This feature is exclusively efficient for the demolition of cancer cells, which cannot survive in the temperature range of 40–48 °C, unlike healthy cells that can endure such temperatures with insignificant or no injury [39][43][44][9,70,71]. It is well known for over three decades that tumor cells have a significant sensitivity to moderate hyperthermia in “fever-range” temperatures (41–45 °C) than normal cells, as usually, the consequences of hyperthermia on healthy cells show up at temperatures >50 °C with coagulation [44][71]. Nanotherm™ (MagForce) consists of amino silane-coated SPIONs and designed for tumor therapy (glioblastoma) using local tissue hyperthermia [45][46][72,73]. Nanotherm™ is already marketed in Europe for the thermotherapy of glioblastoma and is in late-stage clinical trials in the US, and FDA approval is pending [47][48][74,75].
3. Prospects and Conclusions
MMIPs are gaining more interest in the preparation of an efficient targeted DDS especially for cancer treatment and it can be predicted that more imprinted magnetic assisted DDSs will emerge. MMIPs are suitable for in vivo applications due to their superparamagnetic properties, which allows them to show no magnetization after removal of the magnetic field [28][49][59,173]. Their capability to be guided and induce hyperthermia with an appropriate external magnetic field introduces promising ways for cancer treatment, using the MMIPs as smart-drug-delivery robots, a potential alternative to conventional, systemic direct administered chemotherapy [28][59]. To date, these systems are generally investigated in terms of their preparation method stabilization, physicochemical properties, selectivity toward templates, loading capacity, in vitro cytotoxicity, and comparatively simple in vivo tests. The more important issues regarding their safety and side effects, such as the specific interaction of these systems with human organs, tissues, cells or biomolecules, the effect on human’s metabolism brought by the MMIPs, and the wider application of these systems for drug delivery, await further deep study, which should be focused on shortly.
The basis for the creation of a selective strong molecular imprinting of MIPs lies in the formation of stable template-functional monomer adducts in the pre-polymerization reaction mixture. Hence, the choice of functional monomers to form such stable complexes with the template is vital [50][98]. Computational modeling proved itself as a powerful tool for the rational selection of the functional monomers and design of the MMIPs prior to the experiment to prevent waste of time and resources as well as increase the imprinting efficiency. MD simulations have been suggested as a fast method to search for optimal imprinting conditions, especially for the screening of functional monomers.
Nowadays the demand for the development of safe body compatible and degradable DDSs forces the use of such materials in the preparation of delivery systems. As a result, the use of biopolymers like chitosan in the preparation of imprinted systems is getting attention and suggesting further studies to solve the related issues and achieve a controllable efficient system for drug delivery in cancer therapy.
Most published studies have implemented thermally initiated free-radical polymerization in order to synthesize MIPs and MMIPs [30][51][52][61,107,174]. However, photoinitiated free radical polymerization offers several advantages different from thermally initiated free radical polymerization [53][175]. Photoinitiated polymerization is a green method that allows the polymerization to be carried out under more mild conditions, under air, upon blue light exposure, under low light intensity, no need to heat the system and low pressure used. In addition, due to the easiness of turning the light on or off, spatial and temporal control of the initiation step can be reached; such behavior is not possible by heating. Thus, the development of new initiating systems able to initiate polymerization in such conditions is at the center of numerous research [54] [176] due to the low-temperature conditions, facilitation of temporal and spatial control—and especially for practical application solvent-free formulation wavelength flexibility and high curing speed [53][175]. Nevertheless, as mentioned before, photopolymers possibly release residual monomers, photoinitiators, and similar products resulting from decomposition processes into the environment [55][168]. One of the most studied toxic products is tetramethyl succinonitrile (TMSN) that is released from AIBN as the main product of its decomposition and is built into the polymerized plastic product. Animal experiments in rodents have revealed that TMSN could act as a potent convulsant, which leads to the death of animals due to asphyxia [56][177]. Therefore, the choice of a suitable initiator and close monitoring of its residues in the final product as given in the Code of Federal Regulations (CFR), FDA, or other relative regulations is crucial.
To date, the advances in MIT have led to the development of novel synthetically engineered MIPs material that is incorporated with α/β/γ-CDs within an imprinted polymeric framework, which fortunately improved the performance of MIPs [57][58][59][123,124,125] due to their hydrophilic outside and hydrophobic inner space and formation of a non-covalent complex with the guest molecule. Hydrophobic drugs can enter the lipophilic CD cavity with their whole structure or partially. CDs were successfully broad-studied in terms of drug delivery as the functional monomer combined with other functional monomers as binary functional monomers [45][72]. Even the combination of CDs with MIT to generate a DDS is relatively new [18][60][61][49,134,135], it is a highly promising step forward. In one of the reviewed papers, the prepared MMIP containing β-CD as a monomer showed the imprinting ability of this material on the surface of MNPs, high affinity and high adsorption capacity of the imprinted film toward the template and adsorption equilibrium in a short time. These findings are showing the opportunity of further studies with the combination of CDs and MNPs for DDS [61][135].
Furthermore, most NPs in the systemic circulation are recognized by RES and get accumulated in the liver and spleen leading to toxicity to other organs [32][28], suggesting the need for utilizing a stealth approach to overcome this biologic barrier. Poly(ethylene glycol) (PEG) is an FDA approved polymer that has become the most widely used “stealth” polymer in drug delivery. Due to its flexible and hydrophilic nature, on NP surfaces it can form a dynamic hydration barrier, which prevents the plasma proteins binding (also termed opsonization) on the surface of the particles and clearance by the MPS, respectively, [62] [178] suggesting the use of such an approach on the surface of future MMIPs intended for drug delivery.
