You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Jaydira Del Rivero and Version 2 by Yvaine Wei.
Pheochromocytoma and paraganglioma (together PPGL) are rare neuroendocrine tumors that arise from chromaffin tissue and produce catecholamines. Approximately 40% of cases of PPGL carry a germline mutation, suggesting that they have a high degree of heritability. The underlying mutation influences the PPGL clinical presentation such as cell differentiation, specific catecholamine production, tumor location, malignant potential and genetic anticipation, which helps to better understand the clinical course and tailor treatment accordingly. Genetic testing for pheochromocytoma and paraganglioma allows an early detection of hereditary syndromes and facilitates a close follow-up of high-risk patients.
- pheochromocytoma
- paraganglioma
- genetics
- germline
- screening
1. Introduction
Pheochromocytomas (PHEOs) and paragangliomas (PGLs) are rare neuroendocrine (NE) tumors arising from chromaffin cells of the adrenal medulla and extra-adrenal ganglia, respectively. The incidence of PHEOs and PGLs (collectively PPGLs) is estimated at approximately 2–8 cases per million per year [1][2][1,2]. However, this is likely an underestimate, based upon the finding of up to 0.05–0.1% incidentally detected cases in an autopsy series [3]. PPGLs may occur at any age and they usually peak between the 3rd and 5th decade of life [4]. Patients with PPGL most commonly present with symptoms of excess catecholamine production including headache, diaphoresis, palpitations, tremors, facial pallor and hypertension. These symptoms are often paroxysmal, although persistent hypertension between these episodes is common and occurs in 50–60% patients with PPGL [5].
The field of genomics in PPGL has rapidly evolved over the past two decades. Approximately 40% of all cases of PPGLs are associated with germline mutations, which makes pheochromocytoma and paraganglioma solid tumors with a high heritability rate. Currently, more than 20 susceptibility genes have been identified, including at least 12 distinct genetic syndromes, 15 driver genes and an expanding fraction of potential disease modifying genes [6][7][11,12]. Thus, the underlying mutations appear to determine the clinical manifestations, such as tumor location, biochemical profile, malignant potential, imaging signature and overall prognosis, that should help to tailor treatment and guidance for follow-up. Moreover, detection of a mutation in an index case and their family members should also help clinicians to implement a pertinent surveillance program to promptly identify tumors and treat patients accordingly [8][9][13,14]. Surgical resection remains the mainstay of treatment. In cases where surgery is not feasible or if tumor dissemination limits the probability of curative treatment, the options for treatment are localized radiotherapy, radiofrequency or cryoablation and systemic therapy, which includes chemotherapy or targeted molecular therapies.
There has been increasing interest in radionuclide therapy, which includes 131I-MIBG therapy and recently PRRT (peptide receptor radionuclide therapy) 177Lu-DOTATATE [10][11][12][15,16,17]. In terms of chemotherapy, CVD (cyclophosphamide, vincristine and dacarbazine) is one of the most traditional chemotherapy regimens and has been used to treat PPGLs over the past 30 years [13][18]. New treatments are emerging for patients with advanced/metastatic PPGL. Understanding the molecular signaling and metabolomics of PPGL has led to the development of therapeutic regimens for cluster-specific targeted molecular therapies. Based on TCGA classification for cluster I, antiangiogenic therapy, HIF inhibitors, PARP (polyADP-ribose polymerase) inhibition and immunotherapy are used. For cluster II, mTOR (mammalian target of rapamycin) inhibitors are used. Currently there are no cluster III Wnt signaling targeted therapies for PPGL patients [14][19].
At present, clinical genetic testing for patients with a suspected hereditary form of PPGL is carried out using a germline genetic panel rather than using one gene at a time. Based upon its lower financial cost, immunohistochemistry (IHC) can be considered for screening purposes, particularly in patients with suspected succinate dehydrogenase complex (SDHx) mutations. However, IHC should be interpreted with caution as there is likelihood of false-positive and false-negative results [15][20].
2. Genetics on What Is Already Known
The identification of the Krebs cycle in the etiology of PPGLs is a milestone in the field of the genetics of PPGLs. The SDH complex plays a pivotal role in energy metabolism in the Krebs cycle, as well as in complex II of the electron transport chain. Mutations in any of the genes encoding the catalytic enzymes of the pathway can lead to an accumulation of their substrates, resulting in hypoxia-inducible factor (HIF) stability and tumorigenesis [16][22]. These genes include SDHA, SDHB, SDHC, SDHD, SDHAF2 [17][18] [23,24], fumarate hydratase (FH) [19][20][25,26], malate dehydrogenase 2 (MDH2) [21][22][27,28], hypoxia-inducible factor alpha (HIF2a) [23][24][25][29,30,31], prolyl hydroxylase (PHD) [26][32] and some newly discovered genes. Mutation of the genes involved in the kinase receptor signaling pathway that are known to cause PPGLs are RET (REarranged during Transfection), neurofibromin 1 (NF1), Myelocytomatosis-Associated factor X (MAX), transmembrane protein 127 (TMEM127), and Harvey rat sarcoma viral gene homologue (HRAS). Genes such as ATRX (Alpha Thalassemia/mental Retardation-X linked) that are involved in chromosomal integrity, are also implicated as drivers in the etiology of PPGLs and are associated with aggressive behavior [27][33]. To better understand the genetics based on signaling pathways, The Cancer Genome Atlas (TCGA) has classified PPGLs into three clinically useful molecular clusters: (1) Pseudohypoxic PPGLs, (2) Kinase signaling PPGLs and (3) Wnt signaling PPGLs [28][34] (Figure 1).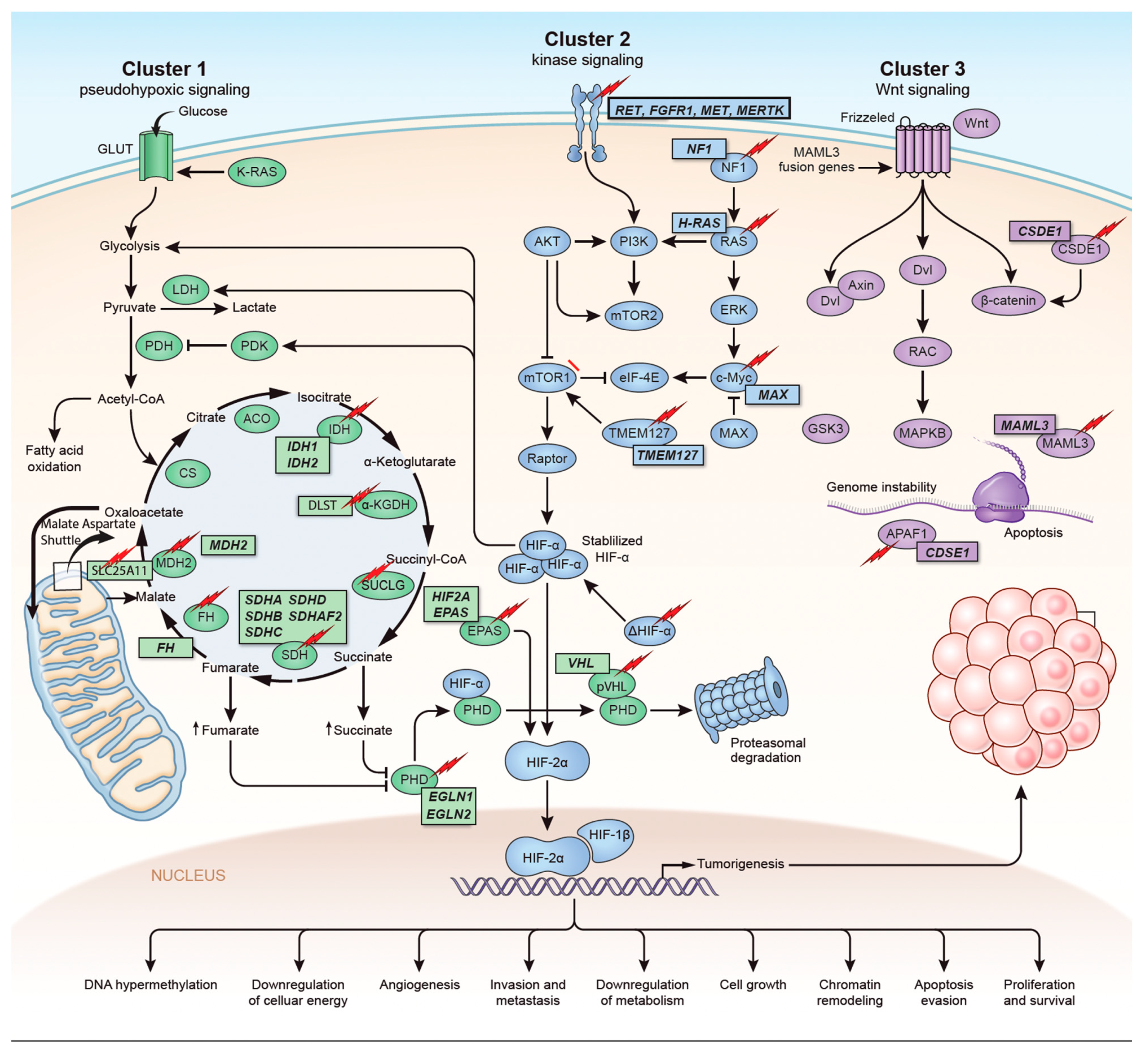
Figure 1. Genetics and molecular pathways for pheochromocytoma and paraganglioma. The genes are classified into three clusters. Cluster I involves mutations in the pseudohypoxic pathway (SDHx, FH, MDH2, HIF2, PHD, VHL and EPAS). Cluster II involves mutations in the kinase signaling group (RET, NF1, TMEM127, MAX and HRAS). Lastly, cluster III includes mutations in the Wnt signaling group (CSDE1 and UBTF fusion at MAML3). The new genes discovered (SUCLG2, SLC25A11, DLST, MAPK, MET, MERTK, FGFR1) have been depicted as well. ↑ depicts accumulation of substrate. Adapted from ref. [19].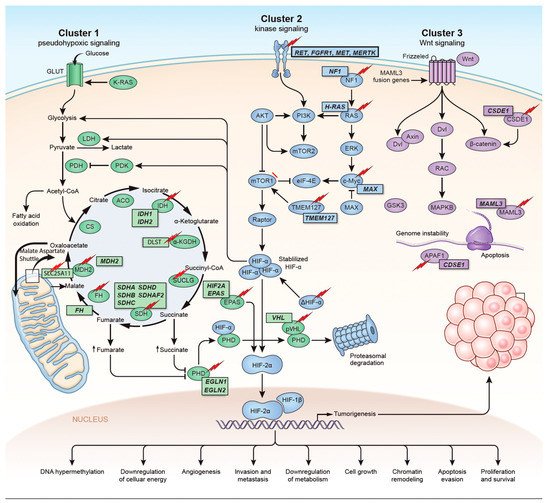

Figure 1. Genetics and molecular pathways for pheochromocytoma and paraganglioma. The genes are classified into three clusters. Cluster I involves mutations in the pseudohypoxic pathway (SDHx, FH, MDH2, HIF2, PHD, VHL and EPAS). Cluster II involves mutations in the kinase signaling group (RET, NF1, TMEM127, MAX and HRAS). Lastly, cluster III includes mutations in the Wnt signaling group (CSDE1 and UBTF fusion at MAML3). The new genes discovered (SUCLG2, SLC25A11, DLST, MAPK, MET, MERTK, FGFR1) have been depicted as well. ↑ depicts accumulation of substrate. Adapted from ref. [19].
3. Genes Discovered in the Last Five Years
With the expanding genetic landscape of PPGLs, several new genes have been identified recently (Table 1) which can potentially predispose patients to the development of tumors with characteristic biological behaviors.
Table 1.
Newly discovered in the pathogenesis of PPGLs.
| Gene | Year of Discovery | Pathophysiology | Gene Type | Metabolomics | References |
|---|---|---|---|---|---|
| CSDE1 | 2016 | Tumor suppressor gene involved in mRNA stability and cellular apoptosis | Somatic | Adrenergic | [29][30][6,7] |
| H3F3A | 2016 | Encodes histone H3.3 protein that regulates chromatin formation | Somatic | NA | [31][32][35,36] |
| MET | 2016 | MAPK signaling pathway | Germline, somatic | NA | [17][23] |
| MERTK | 2016 | Tyrosine kinase receptor | Germline | NA | [6][33][11[34],37,38] |
| UBTF-MAML3 | 2017 | Unique methylation profile mRNA overexpression involved in Wnt receptor and hedgehog signaling pathways | Fusion | Adrenergic | [29][35][6,39] |
| SLC25A11 | 2018 | Encodes malate-oxalate carrier protein of malate-aspartate shuttle | Germline | Noradrenergic | [36][37][40,41] |
| IRP1 | 2018 | Cellular iron metabolism regulation | Somatic | noradrenergic | [38][42] |
| DLST | 2019 | Encodes E2 subunit of mitochondrial α -KG complex which converts α-KG to succinyl-CoA | Germline | Noradrenergic | [17][39][23,43] |
| SUCLG2 | 2021 | Catalyzes conversion of succinyl-coA and ADP/GTP to succinate and ATP/GTP | Germline | Noradrenergic | [40][44] |
