Poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) is thas hie most successful conducting polymer, which has been widely used in displays, transistors, various sensors and photovoltaics (PVs). It has high optical transparency in the visible light range and low-temperature processing condition, making it one of the most widely used polymer hole transport materials inverted perovskite solar cells (PSCs), because of its high optical transparency in the visible light range and low-temperature processing condition. However, the stability of PSCs based on pristine PEDOT:PSS is far from satisfactory, which is ascribed to the acidic and hygroscopic nature of PEDOT:PSS, and property differences between PEDOT:PSS and perovskite materials, such as conductivity, work function and surface morphology. This review summaries recent efficient strategies to improve the stability of PEDOT:PSS in PSCs and discusses the underlying mechanisms. This review is expected to provide helpful insights for further
increasing the stability of PSCs based on commercial PEDOT:PSS.
- PEDOT:PSS
- inverted perovskite solar cells
1. Introduction

2. Methods to Improve the PSCs Stability by Tailoring PEDOT:PSS HTL
|
Method |
Materials |
Perovskite Materials |
PCE (%) |
Stability |
Ref. |
|---|---|---|---|---|---|
|
Doping |
Imidazole |
MAPbI3 |
15.7% |
75% for 14 days, 20% humidity |
[78] |
|
CuSCN /NH3 (aq) |
MAPbI3 |
15.3% |
71% for 175 h |
[79] |
|
|
Ammonia |
MAPbI3-xClx |
13.38% |
90% for 30 days in N2 |
[80] |
|
|
Urea |
MAPbI3 |
18.8% |
97% for 10 days, 35% humidity |
[81] |
|
|
metal oxides |
MAPbI3 |
19.64% |
90% for 45 days in N2, 80% for 20 days in air |
[82] |
|
|
Dopamine |
MAPbI3 |
16.4% |
85.4% for 28 days |
[83] |
|
|
F4-TCNQ |
MAPbI3-xClx |
17.22% |
75% for 150 h, 40% humidity |
[84] |
|
|
DMSO |
MAPbI3 |
16.7% |
83% for 590 h |
[85] |
|
|
Nafion |
MAPbI3 |
16.72% |
86.6% for 500 h, 30–50% humidity |
[86] |
|
|
graphene flakes |
MAPbI3 |
4% |
Stable for one weak |
[87] |
|
|
PSSNa |
MAPbI3 |
15.56% |
>85% for 60 days in N2, |
[88] |
|
|
PFI |
FA0.6MA0.4Sn0.6Pb0.4I3 |
15.85% |
Stable for 300 s |
[89] |
|
|
Triton X-100 |
MAPbI3 |
16.23% |
80% for 500 h |
[90] |
|
|
CTAB |
MAPbI3 |
12.53% |
75% for 30 days, 20–40% humidity |
[91] |
|
|
SBS |
MA0.8FA0.2PbI3-xClx |
19.41% |
90% for 20 days |
[92] |
|
|
EMIC ionic liquid |
MAPbI3 |
20.06% |
85% for 35 days, 60% humidity, 87% after 80 °C for 24 h |
[93] |
|
|
Zn |
MAPbI3 |
13.2% |
91% for 168 h |
[94] |
|
|
RbCl |
MA0.7FA0.3Pb(I0.9Br0.1)3 |
18.3% |
78.17% for 120 h, 50% humidity |
[61] |
|
|
Post-Treatment |
GO |
MAPbI3 |
15.34% |
83.5% for 39 days, 15% humidity |
[95] |
|
WOx doped, EG treated |
MAPbI3Cl3-x |
12.69% |
thermal stable at 250 °C |
[96] |
|
|
EG and MeOH |
MAPbI3 |
18.18% |
65% for 350 h, 45% humidity |
[97] |
|
|
Water |
MAPbI3-xClx |
18.0% |
50% for 240 h in air |
[62] |
|
|
Bilayer |
V2O5 |
MAPbI3 |
15% |
95% for 18 days |
[98] |
|
VOx |
MAPbI3 |
14.22% |
77% for 15 days, 40% humidity |
[99] |
|
|
NPB |
MAPbI3 |
18.4% |
70% for 20 days, 30±5% humidity |
[100] |
|
|
SrGO |
MAPbI3 |
16.01% |
85% for 30 days |
[101] |
|
|
MI |
FA0.2MA0.8PbI3-xClx |
20.68% |
80% for 600 h, 50% humidity |
[102] |
3. Other Methods to Improve the PSCs Stability by Tailoring PEDOT:PSS Layer
References
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792.
- Liao, C.Z.; Zhang, M.; Niu, L.Y.; Zheng, Z.J.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829.
- Liao, C.Z.; Mak, C.H.; Zhang, M.; Chan, H.L.W.; Yan, F. Flexible organic electrochemical transistors for highly selective enzyme biosensors and used for saliva testing. Adv. Mater. 2015, 27, 676–681.
- Agua, I.; Mantione, D.; Ismailov, U.; Sanchez-Sanchez, A.; Aramburu, N.; Malliaras, G.G.; Mecerreyes, D.; Ismailova, E. DVS-crosslinked PEDOT:PSS free-standing and textile electrodes toward wearable health monitoring. Adv. Mater. Technol. 2018, 3, 1700322.
- Fan, X.; Xu, B.G.; Wang, N.X.; Wang, J.Z.; Liu, S.H.; Wang, H.; Yan, F. Highly conductive stretchable all-plastic electrodes using a novel dipping-embedded transfer method for high-performance wearable sensors and semitransparent organic solar cells. Adv. Electron. Mater. 2017, 3, 1600471.
- Mannsfeld, S.C.B.; Tee, B.C.K.; Stoltenberg, R.M.; Chen, C.V.H.H.; Barman, S.; Muir, B.; Sokolov, A.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864.
- Cohen, D.J.; Mitra, D.; Peterson, K.; Maharbiz, M.M. A highly elastic, capacitive strain gauge based on percolating nanotube networks. Nano. Lett. 2012, 12, 1821–1825.
- Sun, J.-Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic Skin. Adv. Mater. 2014, 26, 7608–7614.
- Jeon, J.; Lee, H.; Bao, Z. Flexible wireless temperature sensors based on Ni microparticle-filled binary polymer composites. Adv. Mater. 2013, 25, 850–855.
- Yang, H.; Qi, D.; Liu, Z.; Chandran, B.K.; Wang, T.; Yu, J.; Chen, X. Soft thermal sensor with mechanical adaptability. Adv. Mater. 2016, 28, 9175–9181.
- Gong, S.; Cheng, W.L. One-dimensional nanomaterials for soft electronics. Adv. Electron. Mater. 2017, 3, 1600314.
- Gong, S.; Lai, D.T.H.; Su, B.; Si, K.J.; Ma, Z.; Yap, L.W.; Guo, P.Z.; Cheng, W.L. Highly stretchy black gold e-skin nanopatches as highly sensitive wearable biomedical sensors. Adv. Electron. Mater. 2015, 1, 1400063.
- Chortos, A.; Liu, J.; Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 2016, 15, 937–950.
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.-S.; et al. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013, 12, 938–944.
- Lai, Y.-C.; Deng, J.; Niu, S.; Peng, W.; Wu, C.; Liu, R.; Wen, Z.; Wang, Z.L. Electric eel-skin-inspired mechanically durable and super-stretchable nanogenerator for deformable power source and fully autonomous conformable electronic-skin applications. Adv. Mater. 2016, 28, 10024–10032.
- Xia, Y.; Fang, J.; Li, P.; Zhang, B.; Yao, H.; Chen, J.; Ding, J.; Ouyang, J. Solution-processed highly superparamagnetic and conductive PEDOT:PSS/Fe3O4 nanocomposite films with high transparency and high mechanical flexibility. ACS Appl. Mater. Interfaces 2017, 9, 19001–19010.
- Stapleton, A.J.; Yambem, S.; Johns, A.H.; Gibson, C.T.; Shearer, C.J.; Ellis, A.V.; Shapter, J.G.; Andersson, G.G.; Quinton, J.S.; Burn, P.L.; et al. Pathway to high throughput, low cost indium-free transparent electrodes. J. Mater. Chem. A 2015, 3, 13892–13899.
- Groenendaal, L.; Jonas, F.; Freitag, D.; Peilartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494.
- Cao, Y.; Yu, G.; Menon, R.; Heeger, A.J. Polymer light-emitting diodes with polyethylene dioxythiophene–polystyrene sulfonate as the transparent anode. Synth. Met. 1997, 87, 171–174.
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440.
- Kim, J.Y.; Jung, J.H.; Lee, D.E.; Joo, J. Enhancement of electrical conductivity of poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) by a change of solvents. Synth. Met. 2002, 126, 311–316.
- Ouyang, J.; Xu, Q.; Chu, C.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene): Poly (styrenesulfonate) film through solvent treatment. Polymer 2004, 45, 8443–8450.
- Crispin, X.; Jakobsson, F.L.E.; Crispin, A.; Grim, P.C.M.; Andersson, P.; Volodin, A.; Van Haesendonck, C.; Van der Auweraer, M.; Salaneck, W.R.; Berggren, M. The origin of the high conductivity of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate)(PEDOT-PSS) plastic electrodes. Chem. Mater. 2006, 18, 4354–4360.
- Nardes, A.M.; Janssen, R.A.J.; Kemerink, M.A. A morphological model for the solvent-enhanced conductivity of PEDOT: PSS thin films. Adv. Funct. Mater. 2008, 18, 865–871.
- Döbbelin, M.; Marcilla, R.; Salsamendi, M.; Pozo-Gonzalo, C.; Carrasco, P.M.; Pompos, J.A.; Mecerreyes, D. Influence of ionic liquids on the electrical conductivity and morphology of PEDOT:PSS films. Chem. Mater. 2007, 19, 2147–2149.
- Fan, B.H.; Mei, X.G.; Ouyang, J. Significant conductivity enhancement of conductive poly(3,4-ethylenedioxythiophene): Poly-(styrenesulfonate) films by adding anionic surfactants into polymer solution. Macromolecules 2008, 41, 5971–5973.
- Pettersson, L.A.A.; Ghosh, S.; Inganäs, O. Optical anisotropy in thin films of poly(3,4-ethylenedioxythiophene)-poly(4-styrenesulfonate). Org. Electron. 2002, 3, 143–148.
- Jönsson, S.K.M.; Birgerson, J.; Crispin, X.; Greczynski, G.; Osikowicz, W.; Gon, A.W.D.V.D.; Salaneck, W.R.; Fahlman, M. The effects of solvents on the morphology and sheet resistance in poly(3,4-ethylenedioxythiophene)-polystyrenesulfonic acid (PEDOT-PSS) films. Synth. Met. 2003, 139, 1–10.
- Reyes-Reyes, M.; Cruz-Cruz, I.; Lopez-Sandoval, R. Enhancement of the electrical conductivity in PEDOT: PSS films by the addition of dimethyl Sulfate. J. Phys. Chem. C 2010, 114, 20220–20224.
- Xia, Y.; Ouyang, J. Salt-induced charge screening and significant conductivity enhancement of conducting poly(3,4-ethylenedioxythiophene): Poly (styrenesulfonate). Macromolecules 2009, 42, 4141–4147.
- Xia, Y.; Zhang, H.M.; Ouyang, J. Highly conductive PEDOT: PSS films prepared through a treatment with zwitterions and their application in polymer photovoltaic cells. J. Mater. Chem. 2010, 20, 9740–9747.
- Xia, Y.; Ouyang, J. PEDOT:PSS films with significantly enhanced conductivities induced by preferential solvation with cosolvents and their application in polymer photovoltaic cells. J. Mater. Chem. 2011, 21, 4927–4936.
- Xia, Y.; Ouyang, J. Anion effect on salt-induced conductivity enhancement of poly(3,4-ethylenedioxythiophene): Poly (styrenesulfonate) films. Anion effect on salt-induced conductivity enhancement of poly(3,4-ethylenedioxythiophene): Poly (styrenesulfonate) films. Org. Electron. 2010, 11, 1129–1135.
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT: PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081.
- Xia, Y.; Sun, K.; Ouyang, J. Highly conductive poly(3,4-ethylenedioxythiophene): Poly (styrenesulfonate) films treated with an amphiphilic fluoro compound as the transparent electrode of polymer solar cells. Energy Environ. Sci. 2012, 5, 5325–5332.
- Xia, Y.; Sun, K.; Chang, J.; Ouyang, J. Effects of organic inorganic hybrid perovskite materials on the electronic properties and morphology of poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) and the photovoltaic performance of planar perovskite solar cells. J. Mater. Chem. A 2015, 3, 15897–15904.
- You, J.; Hong, Z.; Yang, Y.; Chen, Q.; Cai, M.; Song, T.B.; Chen, C.C.; Lu, S.; Liu, Y.; Zhou, H.; et al. Low-temperature solution-processed perovskite solar cells with high efficiency and flexibility. ACS Nano 2014, 8, 1674–1680.
- Shao, Y.; Yuan, Y.; Huang, J. Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells. Nat. Energy 2016, 1, 15001.
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525.
- Batmunkh, M.; Vimalanathan, K.; Wu, C.; Bati, A.S.R.; Yu, L.P.; Tawfik, S.A.; Ford, M.J.; Macdonald, T.J.; Raston, C.L.; Priya, S.; et al. Efficient Production of Phosphorene Nanosheets via Shear Stress Mediated Exfoliation for Low-Temperature Perovskite Solar Cells. Small Methods 2019, 3, 1800521.
- McGehee, M.D. Perovskite solar cells: Continuing to soar. Nat. Mater. 2014, 13, 845–846.
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842.
- Beard, M.C.; Luther, J.M.; Nozik, A.J. The promise and challenge of nanostructured solar cells. Nat. Nanotechnol. 2014, 9, 951–954.
- Jean, J.; Brown, P.R.; Jaffe, R.L.; Buonassisi, T.; Bulović, V. Pathways for solar photovoltaics. Energy Environ. Sci. 2015, 8, 1200–1219.
- Wang, K.; Liu, C.; Du, P.; Zheng, J.; Gong, X. Bulk heterojunction perovskite hybrid solar cells with large fill factor. Energy Environ. Sci. 2015, 8, 1245–1255.
- Kim, B.J.; Kim, D.H.; Lee, Y.Y.; Shin, H.W.; Han, G.S.; Hong, J.S.; Mahmood, K.; Ahn, T.K.; Joo, Y.C.; Hong, K.S.; et al. Highly efficient and bending durable perovskite solar cells: Toward a wearable power source. Energy Environ. Sci. 2015, 8, 916–921.
- Bailie, C.D.; Christoforo, M.G.; Mailoa, J.P.; Bowring, A.R.; Unger, E.L.; Nguyen, W.H.; Burschka, J.; Pellet, N.; Lee, J.Z.; Grätzel, M.; et al. Semi-transparent perovskite solar cells for tandems with silicon and CIGS. Energy Environ. Sci. 2015, 8, 956–963.
- Liu, D.; Kelly, T.L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photonics 2014, 8, 133–138.
- Bryant, D.; Greenwood, P.; Troughton, J.; Wijdekop, M.; Carnie, M.; Davies, M.; Wojciechowski, K.; Snaith, H.J.; Watson, T.; Worsley, D. A transparent conductive adhesive laminate electrode for high-efficiency organic-inorganic lead halide perovskite solar cells. Adv. Mater. 2014, 26, 7499–7504.
- Noel, N.K.; Abate, A.; Stranks, S.D.; Parrott, E.S.; Burlakov, V.M.; Goriely, A.; Snaith, H.J. Enhanced photoluminescence and solar cell performance via lewis base passivation of organic–inorganic lead halide perovskites. ACS Nano 2014, 8, 9815–9821.
- Manser, J.S.; Kamat, P.V. Band filling with free charge carriers in organometal halide perovskites. Nat. Photonics 2014, 8, 737–743.
- Chang, J.; Zhu, H.; Li, B.; Isikgor, F.; Hao, Y.; Xu, Q.; Ouyang, J. Boosting the performance of planar heterojunction perovskite solar cell by controlling the precursor purity of perovskite materials. J. Mater. Chem. A 2016, 4, 887–893.
- Chang, J.; Zhu, H.; Xiao, J.; Isikgor, F.; Lin, Z.; Hao, Y.; Zeng, K.; Xu, Q.; Ouyang, J. Enhancing the planar heterojunction perovskite solar cells performance through tuning precursor ratio. J. Mater. Chem. A 2016, 4, 7943–7949.
- Chang, J.; Lin, Z.; Zhu, H.; Isikgor, F.; Xu, Q.; Zhang, C.; Hao, Y.; Ouyang, J. Enhancing the photovoltaic performance of planar heterojunction perovskite solar cells by doping the perovskite layer with alkali metal ions. J. Mater. Chem. A 2016, 4, 16546–16552.
- Zhou, L.; Chang, J.; Liu, Z.; Sun, X.; Lin, Z.; Chen, D.; Zhang, C.; Zhang, J.; Hao, Y. Enhanced planar perovskite solar cell efficiency and stability using a perovskite/PCBM heterojunction formed in one-step. Nanoscale 2018, 10, 3053–3059.
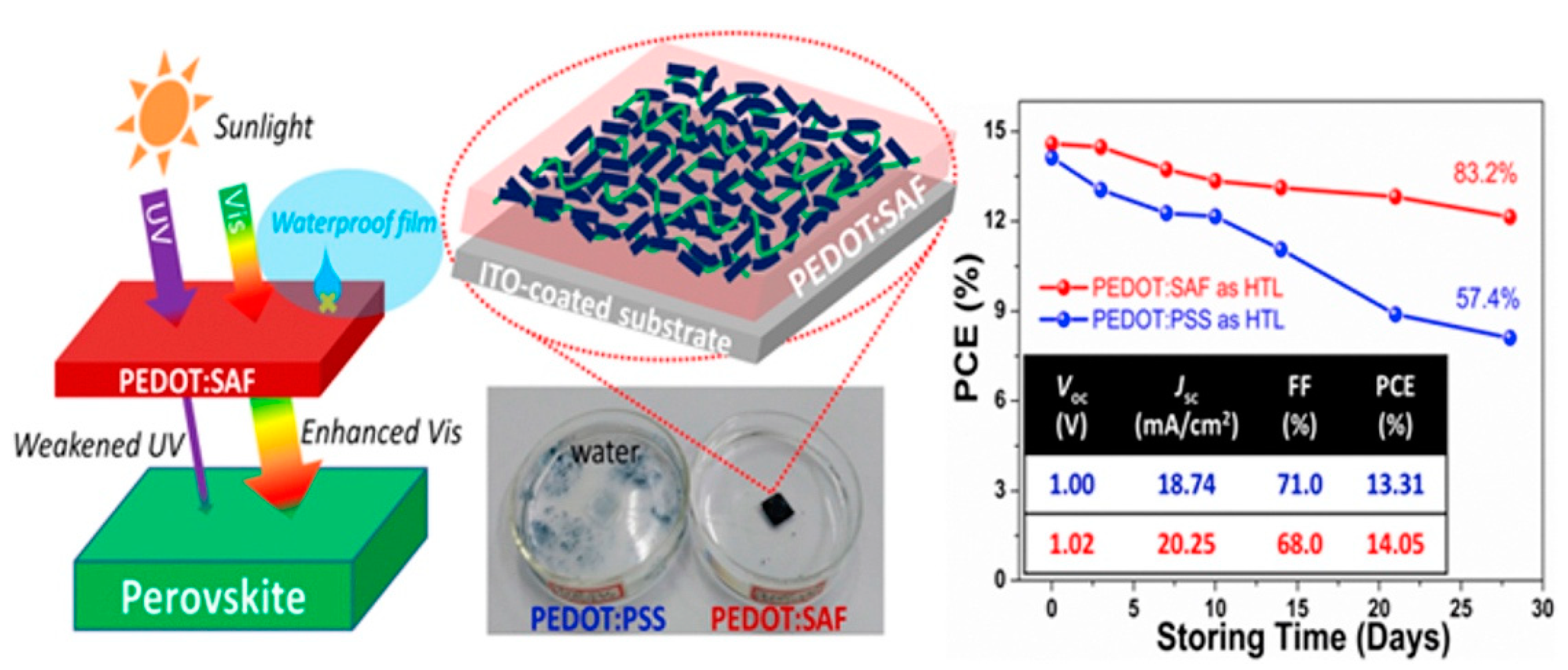
- Yu, W.; Wang, K.; Guo, B.; Qiu, X.; Hao, Y.; Chang, J.; Li, Y. Effect of ultraviolet absorptivity and waterproofness of poly(3,4-ethylenedioxythiophene) with extremely weak acidity, high conductivity on enhanced stability of perovskite solar cells. J. Power Sources 2017, 358, 29–38.
- Huang, J.; Wang, C.; Liu, Z.; Qiu, X.; Yang, J.; Chang, J. Simultaneously enhanced durability and performance by employing dopamine copolymerized PEDOT with high work function and water-proofness for inverted perovskite solar cells. J. Mater. Chem. C 2018, 6, 2311–2318.
- Lin, Z.; Chang, J.; Xiao, J.; Zhu, H.; Xu, Q.; Zhang, C.; Ouyang, J.; Hao, Y. Interface studies of the planar heterojunction perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 157, 783–790.
- Lin, Z.; Zhou, J.; Zhou, L.; Wang, K.; Li, W.; Su, J.; Hao, Y.; Li, Y.; Chang, J. Simultaneously enhanced performance and stability of inverted perovskite solar cells via a rational design of hole transport layer. Org. Electron. 2019, 73, 69–75.
- Hu, L.; Fu, J.; Yang, K.; Xiong, Z.; Wang, M.; Yang, B.; Wang, X.; Tang, X.; Zang, Z. Inhibition of in-plane charge transport in hole transfer layer to achieve high fill factor for inverted planar perovskite solar cells. Sol. RRL 2019, 3, 1900104.
- Liu, X.; Li, B.; Zhang, N.; Yu, Z.; Sun, K.; Tang, B.; Shi, D.; Yao, H.; Ouyang, J. Multifunctional RbCl dopants for efficient inverted planar perovskite solar cell with ultra-high fill factor, negligible hysteresis and improved stability. Nano Energy 2018, 53, 567–578.
- Hu, L.; Li, M.; Yang, K.; Xiong, Z.; Yang, B.; Wang, M.; Tang, X.; Zang, Z.; Liu, X.; Li, B.; et al. PEDOT:PSS monolayers to enhance hole extraction and stability of perovskite solar cells. J. Mater. Chem. A 2018, 6, 16583–16589.
- Hu, L.; Sun, K.; Wang, M.; Chen, W.; Yang, B.; Fu, J.; Xiong, Z.; Li, X.; Tang, X.; Zang, Z.; et al. Inverted planar perovskite solar cells with a high fill factor and negligible hysteresis by the dual effect of NaCl-doped PEDOT: PSS. ACS Appl. Mater. Interfaces 2017, 9, 43902–43909.
- Sun, K.; Li, P.; Xia, Y.; Chang, J.; Ouyang, J. Transparent conductive oxide-free perovskite solar cells with PEDOT: PSS as transparent electrode. ACS Appl. Mater. Interfaces 2015, 7, 15314–15320.
- Zhang, S.; Yu, Z.; Li, P.; Li, B.; Isikgor, F.H.; Du, D.; Sun, K.; Xia, Y.; Ouyang, J. poly(3,4-ethylenedioxythiophene): Polystyrene sulfonate films with low conductivity and low acidity through a treatment of their solutions with probe ultrasonication and their application as hole transport layer in polymer solar cells and perovskite solar cells. Org. Electron. 2016, 32, 149–156.
- Sun, K.; Chang, J.; Isikgor, F.H.; Li, P.; Ouyang, J. Efficiency enhancement of planar perovskite solar cells by adding zwitterion/LiF double interlayers for electron collection. Nanoscale 2015, 7, 896–900.
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051.
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385.
- Hu, L.; Wang, W.; Liu, H.; Peng, J.; Cao, H.; Shao, G.; Xia, Z.; Ma, W.; Tang, J. PbS colloidal quantum dots as an effective hole transporter for planar heterojunction perovskite solar cells. J. Mater. Chem. A 2015, 3, 515–518.
- Syed, A.A.; Poon, C.Y.; Li, H.W.; Zhu, F. A sodium citrate-modified-PEDOT:PSS hole transporting layer for performance enhancement in inverted planar perovskite solar cells. J. Mater. Chem. C 2019, 7, 5260–5266.
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714.
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275.
- Berhe, T.A.; Su, W.N.; Chen, C.H.; Pan, C.J.; Cheng, J.H.; Chen, H.M.; Tsai, M.C.; Chen, L.Y.; Dubale, A.A.; Hwang, B.J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356.
- Adams, J.; Salvador, M.; Lucera, L.; Langner, S.; Spyropoulos, G.D.; Fecher, F.W.; Voigt, M.M.; Dowland, S.A.; Osvet, A.; Egelhaaf, H.J.; et al. Water Ingress in Encapsulated Inverted Organic Solar Cells: Correlating Infrared Imaging and Photovoltaic Performance. Adv. Energy Mater. 2015, 5, 1501065.
- Ye, S.; Sun, W.; Li, Y.; Yan, W.; Peng, H.; Bian, Z.; Liu, Z.; Huang, C. CuSCN-based inverted planar perovskite solar cell with an average PCE of 15.6%. Nano Lett. 2015, 15, 3723–3728.
- You, J.; Meng, L.; Song, T.-B.; Guo, T.-F.; Yang, Y. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nano. 2016, 11, 75–81.
- Christians, J.A.; Fung, R.C.M.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758–764.
- Wang, Q.; Chueh, C.-C.; Eslamian, M.; Jen, A.K.Y. Modulation of PEDOT:PSS pH for Efficient Inverted Perovskite Solar Cells with Reduced Potential Loss and Enhanced Stability. ACS Appl. Mater. Interfaces 2016, 8, 32068–32076.
- Xu, L.; Li, Y.; Zhang, C.; Liu, Y.; Zheng, C.; Lv, W.; Li, M.; Chen, Y.; Huang, W.; Chen, R. Improving the Efficiency and Stability of Inverted Perovskite Solar Cells by Cuscn-Doped Pedot:Pss. Sol. Energy Mater. Sol. Cells 2020, 206, 110316.
- Wang, Y.; Hu, Y.; Han, D.; Yuan, Q.; Cao, T.; Chen, N.; Zhou, D.; Cong, H.; Feng, L. Ammonia-treated graphene oxide and PEDOT:PSS as hole transport layer for high-performance perovskite solar cells with enhanced stability. Org. Electron. 2019, 70, 63–70.
- Elbohy, H.; Bahrami, B.; Mabrouk, S.; Reza, K.M.; Gurung, A.; Pathak, R.; Liang, M.; Qiao, Q.; Zhu, K. Tuning Hole Transport Layer Using Urea for High-Performance Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1806740.
- Duan, C.; Liu, Z.; Yuan, L.; Zhu, H.; Luo, H.; Yan, K. PEDOT:PSS-Metal Oxide Composite Electrode with Regulated Wettability and Work Function for High-Performance Inverted Perovskite Solar Cells. Adv. Opt. Mater. 2020, 8, 2000216.
- Huang, J.; Wang, K.-X.; Chang, J.-J.; Jiang, Y.-Y.; Xiao, Q.-S.; Li, Y. Improving the efficiency and stability of inverted perovskite solar cells with dopamine-copolymerized PEDOT:PSS as a hole extraction layer. J. Mater. Chem. A 2017, 5, 13817–13822.
- Liu, D.; Li, Y.; Yuan, J.; Hong, Q.; Shi, G.; Yuan, D.; Wei, J.; Huang, C.; Tang, J.; Fung, M.-K. Improved performance of inverted planar perovskite solar cells with F4-TCNQ doped PEDOT:PSS hole transport layers. J. Mater. Chem. A 2017, 5, 5701–5708.
- Huang, D.; Goh, T.; Kong, J.; Zheng, Y.; Zhao, S.; Xu, Z.; Taylor, A.D. Perovskite solar cells with a DMSO-treated PEDOT:PSS hole transport layer exhibit higher photovoltaic performance and enhanced durability. Nanoscale 2017, 9, 4236–4243.
- Ma, S.; Qiao, W.; Cheng, T.; Zhang, B.; Yao, J.; Alsaedi, A.; Hayat, T.; Ding, Y.; Tan, Z.A.; Dai, S. Optical-Electrical-Chemical Engineering of PEDOT:PSS by Incorporation of Hydrophobic Nafion for Efficient and Stable Perovskite Solar Cells. ACS. Appl. Mater. Interfaces 2018, 10, 3902–3911.
- Redondo-Obispo, C.; Ripolles, T.S.; Cortijo-Campos, S.; Lvarez, A.L.; Coya, C. Enhanced stability and efficiency in inverted perovskite solar cells through graphene doping of PEDOT: PSS hole transport layer. Mater. Des. 2020, 191, 108587–108618.
- Zuo, C.T.; Ding, L.M. Modified PEDOT layer makes a 1.52 V VOC for perovskite/PCBM solar cells. Adv. Energy Mater. 2016, 7, 1601193.
- Tang, H.; Shang, Y.; Zhou, W.; Peng, Z.; Ning, Z. Energy level tuning of PEDOT:PSS for high performance tin-lead mixed perovskite solar cells. Sol. RRL 2019, 3, 1800256.
- Shin, D.; Kang, D.; Lee, J.-B.; Ahn, J.-H.; Cho, I.-W.; Ryu, M.-Y.; Cho, S.W.; Jung, N.E.; Lee, H.; Yi, Y. Electronic Structure of Nonionic Surfactant-Modified PEDOT:PSS and Its Application in Perovskite Solar Cells with Reduced Interface Recombination. ACS Appl. Mater. Interfaces 2019, 11, 17028–17034.
- Zhu, Y.; Wang, S.; Ma, R.; Wang, C. The improvement of inverted perovskite solar cells by the introduction of CTAB into PEDOT:PSS. Sol. Energy 2019, 188, 28–34.
- Li, W.; Wang, H.; Hu, X.; Cai, W.; Zhang, C.; Wang, M.; Zang, Z. Sodium Benzenesulfonate Modified Poly (3,4-Ethylenedioxythiophene): Polystyrene Sulfonate with Improved Wettability and Work Function for Efficient and Stable Perovskite Solar Cells. Sol. RRL 2021, 5, 2000573.
- Zhou, X.; Hu, M.; Liu, C.; Zhang, L.; Zhong, X.; Li, X.; Tian, Y.; Cheng, C.; Xu, B. Synergistic effects of multiple functional ionic liquid-treated PEDOT:PSS and less-ion-defects S-acetylthiocholine chloride-passivated perovskite surface enabling stable and hysteresis-free inverted perovskite solar cells with conversion efficiency over 20%. Nano Energy 2019, 63, 103866.
- Hamed, M.A.; Fatma, P.G.C.; Furkan, K.; Ayse, E.; Serap, G. Improvement of fill factor by the utilization of Zn-doped PEDOT:PSS hole-transport layers for p-i-n planar type of perovskite solar cells. Electrochim. Acta 2021, 388, 138658.
- Luo, H.; Lin, X.; Hou, X.; Pan, L.; Huang, S.; Chen, X. Efficient and air-stable planar perovskite solar cells formed on graphene-oxide-modified PEDOT:PSS hole transport layer. Nano Lett. 2017, 9, 19–29.
- Kanwat, A.; Rani, V.; Jang, J. Improved power conversion efficiency of perovskite solar cells using highly conductive WOx doped PEDOT: PSS. Improved power conversion efficiency of perovskite solar cells using highly conductive WOx doped PEDOT: PSS. New J. Chem. 2018, 42, 16075–16082.
- Reza, K.M.; Gurung, A.; Bahrami, B.; Mabrouk, S.; Elbohy, H.; Pathak, R.; Chen, K.; Chowdhury, A.H.; Rahman, M.T.; Letourneau, S.; et al. Tailored PEDOT:PSS hole transport layer for higher performance in perovskite solar cells: Enhancement of electrical and optical properties with improved morphology. J. Energy. Chem. 2020, 44, 41–50.
- Wang, D.; Elumalai, N.K.; Mahmud, M.A.; Wright, M.; Upama, M.B.; Chan, K.H.; Xu, C.; Haque, F.; Conibeer, G.; Uddin, A. V2O5-PEDOT:PSS bilayer as hole transport layer for highly efficient and stable perovskite solar cells. Org. Electron. 2018, 53, 66–73.
- Xu, L.G.; Qian, M.Y.; Lu, Q.; Zhang, H.M.; Huang, W. Low temperature processed PEDOT:PSS/VOx bilayer for hysteresis-free and stable perovskite solar cells. Mater. Lett. 2019, 236, 16–18.
- Ma, S.; Liu, X.; Wu, Y.; Tao, Y.; Ding, Y.; Cai, M.; Dai, S.; Liu, X.; Alsaedi, A.; Hayat, T. Efficient and flexible solar cells with improved stability through incorporation of a multifunctional small molecule at PEDOT:PSS/perovskite interface. Sol. Energy Mater. Sol. Cells 2020, 208, 110379.
- Mann, D.S.; Seo, Y.-H.; Kwon, S.-N.; Na, S.-I. Efficient and stable planar perovskite solar cells with a PEDOT:PSS/SrGO hole interfacial layer. J. Alloy. Compd. 2020, 812, 152091.
- Wang, M.; Li, W.; Wang, H.; Yang, K.; Hu, X.; Sun, K.; Lu, S.; Zang, Z. Small Molecule Modulator at the Interface for Efficient Perovskite Solar Cells with High Short-Circuit Current Density and Hysteresis Free. Adv. Electron. Mater. 2020, 6, 2000604.
- Jiang, X.; Yu, Z.; Zhang, Y.; Lai, J.; Li, J.; Gurzadyan, G.G.; Yang, X.; Sun, L. High-Performance Regular Perovskite Solar Cells Employing Low-Cost Poly(ethylenedioxythiophene) as a Hole-Transporting Material. Sci. Rep. 2017, 7, 42564.
- Erazo, E.A.; Daniel, C.B.; Pablo, O.; María, T.C. NaCl doped electrochemical PEDOT:PSS layers for inverted perovskite solar cells with enhanced stability. Synthetic Met. 2019, 257, 116178.
- Chen, W.-H.; Qiu, L.; Zhang, P.; Jiang, P.-C.; Du, P.; Song, L.; Xiong, J.; Ko, F. Simple fabrication of a highly conductive and passivated PEDOT:PSS film via cryocontrolled quasi-congealing spin-coating for flexible perovskite solar cells. J. Mater. Chem. C 2019, 7, 10247–10256.
- Tsai, T.-C.; Chang, H.-C.; Chen, C.-H.; Huang, Y.-C.; Whang, W.-T. A Facile Dedoping Approach for Effectively Tuning Thermoelectricity and Acidity of PEDOT:PSS Films. Org. Electron. 2014, 15, 641–645.
- Tehrani, P.; Kanciurzewska, A.; Crispin, X.; Robinson, N.; Fahlman, M.; Berggren, M. The Effect of Ph on the Electrochemical over-Oxidation in PEDOT:PSS Films. Solid State Ionics 2007, 177, 3521–3527.
- Chen, S.; Song, L.; Tao, Z.; Shao, X.; Huang, Y.; Cui, Q.; Guo, X. Neutral-Ph PEDOT:PSS as over-Coating Layer for Stable Silver Nanowire Flexible Transparent Conductive Films. Org. Electron. 2014, 15, 3654–3659.
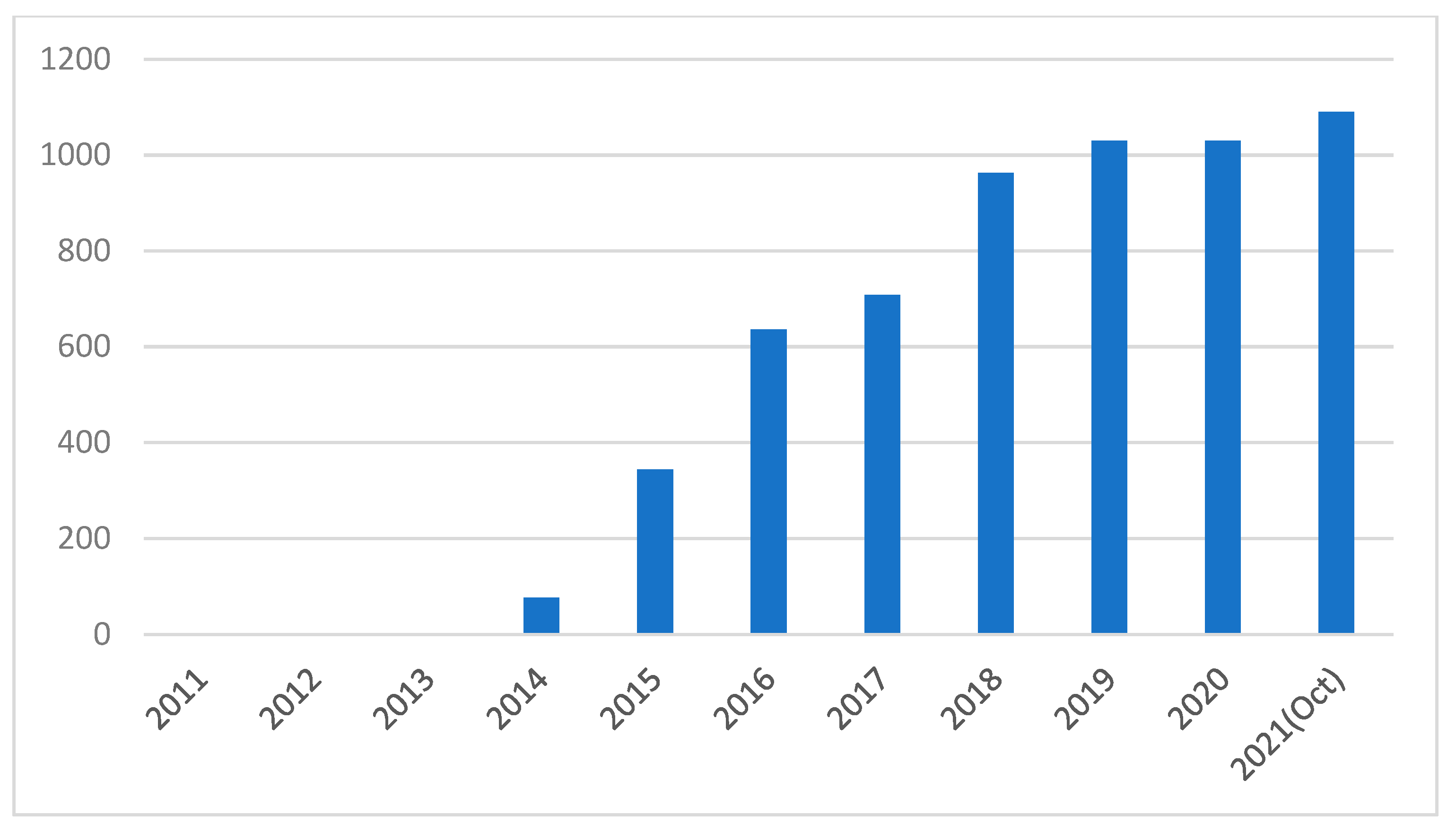
- Available online: https://scholar.google.com.hk/scholar?hl=zh-CN&as_sdt=0%2C5&q=PEDOT%3APSS%2C+perovskite+solar+cells+&btnG= (accessed on 21 October 2021).
- Chen, J.; Zhang, J.; Huang, C.; Bi, Z.; Yu, H.; Shi, S.; Xu, X. Two-dimensional Bi2OS2 doping improves the performance and stability of perovskite solar cells. Chem. Eng. J. 2020, 420, 127700.
- Ibrahim, K.; Shahin, A.; Jones, A.; Alshehri, A.H.; Mistry, K.; Singh, M.D.; Ye, F.; Sanderson, J.; Musselman, K.P. Humidity-resistant perovskite solar cells via the incorporation of halogenated graphene particles. Sol. Energy 2021, 224, 787–797.
- Yang, H.; Liu, N.; Ran, M.; He, Z.; Meng, R.; Chen, M.; Lu, H.; Yang, Y. Enhancing electron transport in perovskite solar cells by incorporating GO to the meso-structured TiO2 layer. J. Mater. Sci. Mater. Electron. 2020, 31, 3603–3612.
