Folates are crucial to life, as a component and catalyst for essential biochemical reactions, particularly and especially via their central role in the metabolism of nucleotides for DNA synthesis and methylation processes involved in imprinting and epigenesis.involved in imprinting and epigenesis
1. Introduction
Folates are intermediary metabolites in the folate cycle (FC), which is linked to and supports the one carbon cycle (1-CC). Together, these two metabolic pathways are responsible for generating methyl groups and regulating all processes involved in methylation, and thus epigenetic modifications and imprinting. The two cycles are also directly or indirectly implicated in numerous other linked metabolic processes that regulate cell division and tissue development; maintaining the appropriate balance of substrates and cofactors is essential for correct homeostasis, as disruption of an enzymatic step in either cycle can have significant adverse consequences. This is particularly important during early pregnancy, where folate deficiency has an impact on neurodevelopment and placental growth. Altered methylation patterns in placental genes have an effect on fetal growth and development.
5-MTHF is the main form of dietary folate, and represents the predominant physiologic form of folate found in blood; the availability of 5-MTHF contributes to the conversion of methionine and and then then to SAM (S-adenosylmethionine), the universal effector for methylation. After the release of a methyl group, S-adenosylhomocysteine (SAH) and homocysteine accumulation exert feedback methylatuion inhibition . A number of consecutive steps in the two cycles are subject to mutations due to single nucleotide polymorphisms (SNPs) hat affect the efficiency of the cycles by decreasing MTHFR activity, compromising methylation reactions via the reduced availability of methionine, and the accumulation of homocysteine.
Folic acid (FA) administered as a dietary supplement is a synthetic compound, and its metabolism requires initial reduction by DHFR in the liver
[1][2]. This enzyme has weak activity, and in conjunction with SNPs that impair the activity of MTHFR, nutritional supplementation with FA can lead to a syndrome now recognized as UMFA: unmetabolized folic acid syndrome. Detectable levels of UMFA occur temporarily in plasma after the consumption of >200 µg FA, with concentrations increasing parallel to that of total FA after supplementation.
2. The Folate Family
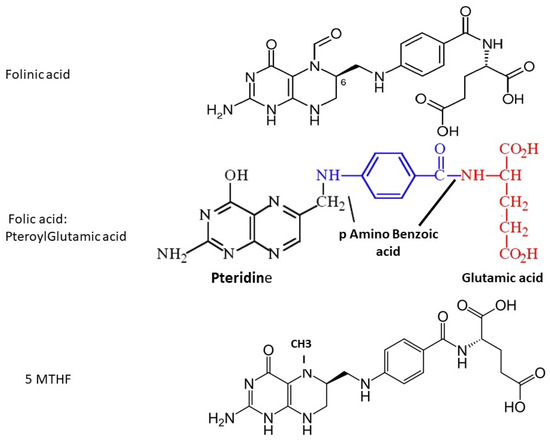
The term ‘folate’ includes several different forms, all of which contain a pteroyl group (see
Figure 1). Naturally-occurring folate (Vitamin B9) is a water-soluble molecule that exists physiologically as tetrahydrofolate (THF, the active form) and methyltetrahydrofolate (MTHF, primary form found in blood). Folic acid (FA) is a synthetic manufactured molecule that is used as a dietary supplement and in foodstuff fortification. It is fully oxidized, and is not present in blood unless ingested in food or supplements. Its biological activity depends on the action of dihydrofolate reductase (DHFR) enzyme in the liver, which has unusually slow activity in humans. In order to fulfill a physiological function by entering the folate cycles, FA must first be reduced by DHFR to DiHydroFolate (DHF), and then to tetrahydofolate (THF), before it is converted to the biologically active 5-MTHF.
Figure 1. Molecular structure of folinic acid, folic acid and 5-methyltetrahydrofolate; fluorescence-based assays measure the pteroyl group, which is common to all three molecules.
Folinic acid (Leucovorin) is also a synthetic molecule, a 5-formyl derivative of THF that is readily converted to 5–10 MTHF and 5-MTHF without requiring the action of DHFR. It is used to decrease the toxic effects of chemotherapeutic agents that interfere with folate metabolism by inhibiting DHFR (e.g., methotrexate), and in the co-treatment of other pathologies treated with anti-folate drugs.
2.1. Folate Levels and Folate Assays
Assays that measure folate concentrations in blood are subject to major issues that are often neglected
[2][4]. Fluorescence-based assays measure all “folates” with a pteroyl core, and this includes UMFA, as well as THF, DHF, methyleneTHF, 5-MTHF and FA. When fluorescence assays are used to monitor folate levels after FA is prescribed, “folate” levels are seen to increase, irrespective of their true formula. The results are therefore meaningless, since they do not measure biologically active folates and do not reflect a true physiological status. Assays that are based upon liquid chromatography (+/− mass spectrometry) can measure the three nutraceutical complements that may be prescribed (
Figure 1): folinic acid (FLA), folic acid (FA, pteroylglutamic acid) and 5-MTHF, and these should be used in order to determine physiological folate status.
3. Metabolism of Synthetic Folic Acid (Pteroylglutamic Acid, FA) and the UMFA Syndrome
In order to enter the folate cycle, FA must first be converted to tetrahydrofolate (THF) via two reducing biochemical steps catalyzed by DHFR—dihydrofolate reductase, (co-factor NADP(H)). This is a rate-limiting step, and DHFR has very weak activity in humans even in the absence of SNP mutations, with considerable inter-individual variation. Therefore, experiments carried out in animal systems (especially rats, where activity can be estimated as 25× higher than in humans), should take this specificity into account. High doses of FA leads to a rapid saturation/inhibition of the DHFR enzyme, leading to an accumulation of un-metabolized folic acid (UMFA) and the UMFA syndrome
[1][2]. These authors confirm that the capacity to metabolize folic acid in humans is low, especially at high FA doses (5 mG or more); the efficacy of prescribing such high doses is questionable, as this may be associated with several pathological issues. Levels of circulating UMFA
(unmetaboli
zed folic acid) in the population is persistent in countries where the FA fortification of grains and cereals is implemented
[3][14]. In the BBC (Boston Birth Control) cohort, UMFA was always detected at birth
[4][15]. UMFA may compete with natural folate (5-MTHF) for the folate transporter (SLC19A1) and the folate receptor (FolR1), thus depleting active folate for participation in the two metabolic cycles. The PCFT-SLC46A1, proton-coupled folate transporter, responsible for transport of folates in the intestine, at low pH; is not detected/active in the early embryos, where all the fluids are at an alkaline bicarbonate-mediated pH. However, this transporter is expressed in fetal tissues as early as ten weeks gestation, at high levels in the intestine of a 20-week old fetus, and in the placenta, and this could be a factor in the appearance of UMFA in cord blood at birth. Tetrahydrofolate (THF) produced by DHFR is converted to 5–10 methylene THF by methylene tetrahydrofolate dehydrogenase (MTHFD1), without specific problems. However, five rare but significant SNPs that affect this step have been described in association with cancers, migraines, congenital anomalies such as neural tube defects, and congenital heart disease
[5][16]. The most hazardous mutation appears to be G1958A. THF/5–10 methylene THF accumulates at the level of MTHFR, and the accumulation of unmetabolized folates upstream from the enzyme may lead to competitive inhibition, leading to Hcy accumulation
[6][7][17,18]. MTHFR is allosterically inhibited by SAM, and this also means that an excess of 5-MTHF may be deleterious and that circulating Met level is controlled. This level of fine-tuned regulation must be respected: a high consumption of FA may lead to a pseudo- MTHFR deficiency in healthy patients.
4. Folinic Acid (5 Formyl THF, FLA)
Folinic acid binds the classical receptor FolR1 and is transported into cells by the solute carrier SLC19A1. Its metabolism leads to the formation of THF and 5, 10 Methylene THF; an important part of these metabolic steps allows the formation of thymidylate, and the failure of this pathway in folate deficiency leads to DNA damage that has been associated with carcinogenesis
[8][1]. Purine nucleotide biosynthesis de novo (PNB) requires two folate-dependent transformylases utilizing formylTHF. FLA is sometimes prescribed as a treatment for problems associated with MTHFR SNPs, but its metabolites do not succeed in compensating for low MTHFR activity. FLA prevents UMFA accumulation by bypassing weak DHFR activity.
5. 5 Methyl THF
5 methyl THF is the natural folate which does not induce UMFA; It is present in "green" vegetable. It The efficiency and safety of 5-MTHF has been established, including in children , and has been demonstrated to be at least as efficient as FA in reducing homocysteine levels in healthy women . It is effective in reducing Hcy in men and women of reproductive age who are of carriers of the MTHFR T677T variant (31-38). 5-methylTHF can/could effectively prevent NTDs by improving folate biomarkers in young women during early pregnancy . No large-scale clinical studies have been performed yet. 250+ pregnancies have started, followed by deliveries, with MTHF treatment at a daily dose of 600-800 µG per day . This includes women having previously suffered NTDs or miscarriages with FA at 5 mG/Day. 5MTHF supply could be a better alternative to FA in reducing the incidence of NTD, especially in countries that do not implement a program of FA fortification. It is at least as effective before and during pregnancy. Sperm of men carrying MTHFR SNPs have a significant impact on the cytogenetic quality of early embryos, (with a subsequent increased risk of miscarriage (48,49). A similar feature can be seen for women. 5-MTHF can provide a solution for problems related to folate cycle metabolism : 5-MTHF supplementation can overcome MTHFR SNPs in patients who have failed to conceive even after treatment with high doses of FA, 5 to 15 mg per day.
In conlusion 5 MTHF seems to be a better option for the support of "folate. " in population needing it. This is especially true in MTHFR SNP carrriers aither men and women. UMFA is a source of concern