Researchers already improved the properties of β-TCP by achieving optimum surface and bulk β-TCP chemical/physical properties through the hydrothermal addition of magnesium (Mg) and to later establish the biocompatibility of β-TCP/Mg for bone grafting and tissue engineering treatments. The present results indicate that the hydrothermal addition of 1.4 wt% MgO to the particle surface of β- TCP particle significantly increased cell proliferation and osteoblastic differentiation in vitro and resulted in more new bone regeneration from histologic and micro-CT evaluation in vivo compared to the β-TCP control particles; altogether, Mg was advantageous to commercial β-TCP bone regeneration.
We already improved the properties of β-TCP by achieving optimum surface and bulk β-TCP chemical/physical properties through the hydrothermal addition of magnesium (Mg) and to later establish the biocompatibility of β-TCP/Mg for bone grafting and tissue engineering treatments.
The present results indicate that the hydrothermal addition of 1.4 wt% MgO to the particle surface of β- TCP particle significantly increased cell proliferation and osteoblastic differentiation in vitro and resulted in more new bone regeneration from histologic and micro-CT evaluation in vivo compared to the β-TCP control particles; altogether, Mg was advantageous to commercial β-TCP bone regeneration.
1. Introduction
In the dental field, the treatment of pathological conditions such as periodontitis, traumatisms, and oral cancer can lead to different types of bone defects. For example, the results of a radiographic evaluation showed that the prevalence and distribution of bone defects were linked to chronic periodontitis. In addition, the authors found that the prevalence of bone defects was over 80% and that bone loss was higher in males than in females
[1]. Oral cancer is the sixth most common malignancy worldwide
[2]. However, root fractures are responsible for only 0.5%–0.7% of all dentoalveolar cases in permanent dentition and are frequently accompanied by fractures of the alveolar bone. The restoration of these anterior dental traumas in young adults can be complex
[3]. Even though oral cancer is not prevalent compared to other tumor entities, the condition generates significant mortality and morbidity in patients, specifically when discovered in the delayed stages of the disease
[4]. When oral cancer is successfully treated, the treatment of bone defects becomes necessary. All of these bone defects can be treated with autografts, which are still considered the gold standard and are most used type of bone graft
[5]. However, autografts have several drawbacks, including a limited supply and additional surgery to obtain the graft, with possible donor-site morbidity
[5]. The above-mentioned obstacles can be avoided by using other types of bone grafts, such as biocompatible and bioresorbable alloplastic pure-phase β-TCP. With β-TCP, particle sizes that vary between 500 and 1000 μm with 65% porosity can improve osteoblastogenesis by ameliorating the capillary effect of the blood, which stimulates new bone regeneration by osteoconduction over a period of between 4 and 12 months
[6]. Nowadays, β-TCP is used in a range of bone surgeries in dentistry and medical orthopedics
[7][8][9][7,8,9].
In recent years, the particular concentration of magnesium (Mg) ions has been found to play a pivotal role in new bone formation and regeneration. The addition of Mg ions is a simple yet effective method to enhance the osteogenesis and angiogenesis of bio-ceramic scaffolds and to ameliorate the evolution of biomaterials and bone tissue engineering scaffolds
[10]. However, Mg can trigger an acute and unfavorable inflammatory response that can lead to the destruction of adjacent normal bone and the formation of fibrous tissue.
[11]. In a more recent review article, the addition of Mg
2+ to BCP was reported to lead to desirable physicochemical properties that could be observed in both in vitro and in vivo results, suggesting that Mg-doped biphasic calcium phosphate (Mg-BCP) is biocompatible and efficient as a bone substitute. Despite these promising outcomes, similar to other biomaterials for bone regeneration, the optimal physicochemical properties must be established. Further investigations are required regarding Mg-BCP applications in bone tissue engineering
[12].
In daily dental practice, to enhance bone tissue engineering approaches for the various types of bone defects and to achieve the best surface and bulk material properties, the β-TCP and MgO synthesis method should be chosen carefully
[13]. Having the ability to create crystalline phases that are not stable at the melting point, hydrothermal synthesis, also known as the hydrothermal method, can be used to synthesize crystals that depend on the solubility of minerals in hot water under high pressure
[14]. This method has proven to be a suitable Mg-doping method at the phase transformation temperature of β-TCP ceramics
[15]. However, minimal modifications in the sintering process of β-TCP can lead to different properties that ultimately can undermine results, such as new bone regeneration, when used for bone defect treatments
[7].
2. Physicochemical Properties of β-TCP/Mg
The SEM results show that the β-TCP/Mg particles exhibited Mg on their surfaces while maintaining the similar roughness and morphology of the β-TCP control particle structures (
Figure 1).
Figure 1. Scanning electron microscope images of the surface view of the obtained hydrothermally treated vs. nontreated β-TCP. (A) β-TCP/Mg apparent fusion of MgO ions with the β-TCP surface is observed. (B) β-TCP control.
In vitro, the EDS results demonstrated that a 1.4 weight percentage of Mg was uniformly distributed on the β-TCP/Mg surface while maintaining a 19.7 wt% P and slightly inferior 35.9 wt% Ca compared to the 20.8 wt% P and 39.2 wt% Ca found in the control β-TCP (
Table 1).
Table 1. Elemental results by EDS (Energy-Dispersive X-ray Spectrometry).
| Chemical Element |
β-TCP/Mg (Test) |
β-TCP (Control) |
| Weight (%) |
Weight (%) |
| Mg |
1.4 |
0.0 |
| Ca |
35.9 |
39.2 |
| P |
19.7 |
20.8 |
| O |
43.0 |
40.1 |
| Ca |
0.0 |
0.0 |
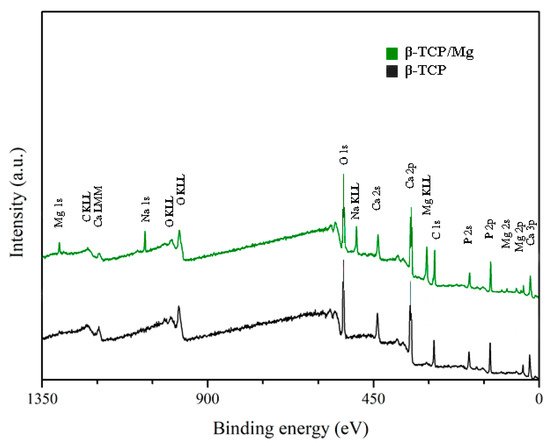
The XPS results exhibited no statistically significant differences between the Ca/P ratio of β-TCP/Mg particles (1.40) and that of β-TCP particles (1.38). However, both β-TCP particles had slightly lower Ca/P ratios than the previously described 1.5 stoichiometric TCP ratio
[16][17]. The (O1s) mean value for β-TCP/Mg was 48.43%, and that for the β-TCP control was 49.74%. The values for the β-TCP control and β-TCP/Mg samples were 11.80% and 8.79% (P2p), respectively. At the same time, the Ca2p values were 12.36% in β-TCP/Mg and 16.36% in the β-TCP control (
Table 2;
Figure 2).
Figure 2. XPS spectra. Untreated beta-TCP and beta-TCP/Mg fragments reveal Ca, P, and O. Limited quantities of Mg were present in the β-TCP/Mg samples.
Table 2. XPS analyses (%).
| Group |
O1s |
Na1s |
Mg1s |
Si2p |
P2p |
S2p |
Ca2p |
Mg/Ca |
P/Ca |
P/Ca + Mg |
| β-TCP/Mg |
48.43 |
3.67 |
2.08 |
0.74 |
8.79 |
0.39 |
12.36 |
16.80% |
71.10% |
60.90% |
| β-TCP |
49.74 |
|
|
|
11.8 |
0.83 |
16.36 |
0.00% |
72.10% |
72.10% |
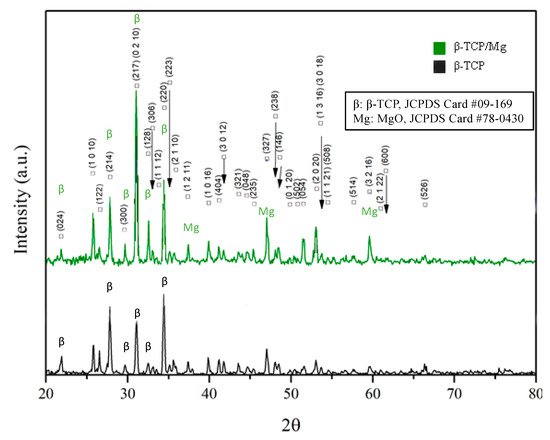
Figure 3 Illustrates that MgO incorporation had no significant effect on the lattice parameters detected by XRD in the β-TCP structure, contrary to what was found in a study where Mg
2+ was doped at the phase transformation temperature of β-TCP ceramics. Nevertheless, the β-TCP/Mg showed diffraction patterns corresponding to the β-TCP (JCPDS file no. 09-169, International Center for Diffraction Data) and MgO reference peaks (JCPDS file no. 78-0430, International Center for Diffraction Data), indicating that the MgO powders were well-incorporated on β-TCP graft’s surface overall.
Figure 3. X-ray diffraction patterns of β-TCP/Mg and β-TCP control particles generating the same intense sharp-peak samples.
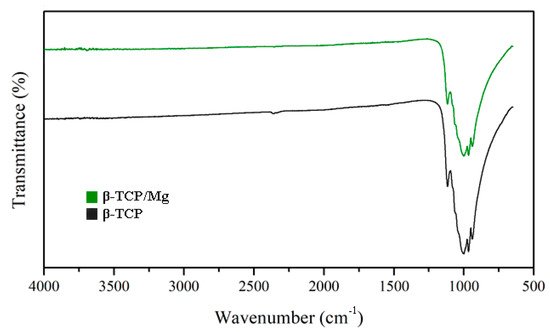
The FTIR results reinforced the XRD findings by presenting similar high-intensity peaks at the 1043 and 1120 cm
−1 absorption bands in the β-TCP/Mg and untreated β-TCP particles (
Figure 4).
Figure 4.
Beta-TCP/Mg and beta-TCP control have identical FTIR spectra absorption peak heights.
3. In Vitro Biocompatibility
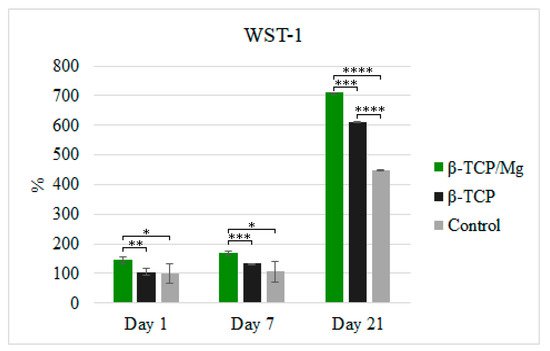
The in vitro cytotoxicity and proliferation of MG-63 at 1, 7, and 21 days in the β-TCP/Mg and untreated β-TCP media behaved according to ISO10993-5. The results are shown in
Figure 5. The percentages of cells cultivated in the media with the β-TCP/Mg particles were 144.45%, 168.92%, and 709.88% on days 1, 7, and 21, respectively. Conversely, MG-63 proliferation on the nontreated β-TCP was 105.22%, 132.84%, and 611.90% on days 1, 7, and 21, respectively.
Figure 5. MG-63 cell proliferation measured using WST-1 on days 1, 7, and 21. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, and **** p ≤ 0.0001.
β-TCP/Mg improved MG-63 cell proliferation and displayed excellent biocompatibility with a statistically significant difference compared to the cells cultured with nontreated β-TCP and control cells (
Figure 5). However, the cells cultured with untreated β-TCP had proliferated significantly more than control cells (
p < 0.0001) by day 21. Additionally, when phalloidin/DAPI immunofluorescence staining was used, it was determined that the cells on β-TCP control and β-TCP/Mg proliferated and presented with a spindle shape and filopodial extensions (
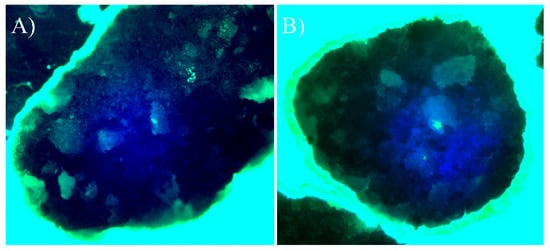
Figure 6).
Figure 6. Direct cell culture with DAPI (4′,6-diamidino-2-phenylindole)/phalloidin fluorescent imaging at 20× after 24 h. (A) Beta-tricalcium phosphate; (B) Beta-tricalcium phosphate/Mg.
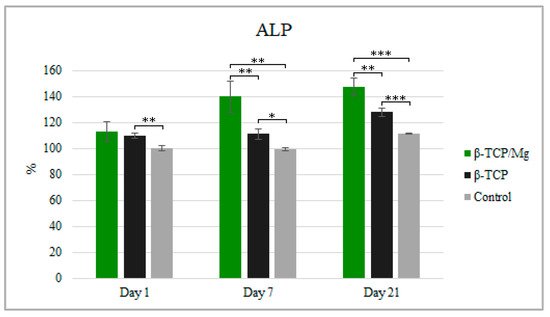
Despite having significantly more cell proliferation, as observed in WST-1, the ALP test showed MG-63 cultivation with β-TCP/Mg, with 112.92% cultivation at 1 day, 139.95% cultivation at day 7, and 147.37% cultivation at day 21 when compared to the number of cells in the β-TCP/medium or in the medium alone after 21 days of culture. Furthermore, the cells produced higher ALP when cultured in the β-TCP/Mg medium than when cultured in the β-TCP control or in medium alone at 21 days (
p < 0.05;
Figure 7).
Figure 7. ALP activity of MG-63 after being cultured for 1, 7, and 21 days with the β-TCP/Mg, untreated β-TCP, or just DMEM as control. * p ≤ 0.05, ** p ≤ 0.01, and *** p ≤ 0.001.
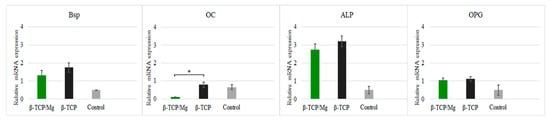
Nonetheless, high concentrations of Mg ions reduced osteoblast differentiation and inhibited the expression of specific osteogenic markers
[17][18], as was seen with the gene expression in this study (
Figure 8).
Figure 8. Relative expression BSP, OC, ALP, and OPG of MG-63 cells maintained in beta-tricalcium phosphate/Mg, beta-tricalcium phosphate, or just DMEM (control). * p ≤ 0.05.
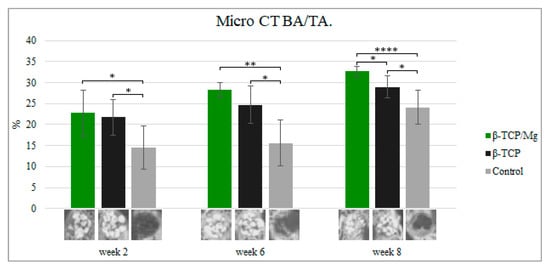
In vivo, the β-TCP/Mg particle grafts started creating new bone while degrading critical calvaria size defect healing in the New Zealand white rabbits, as seen in micro-CT results, from week 2 to week 7.
During week 2, new bone formation defects in the calvarial region packed with untreated β-TCP, β-TCP/Mg, and control regenerated 21.74% ± 4.24%, 22.73% ± 5.39%, and 14.54% ± 5.23%, respectively. The defects that were treated with beta-TCP control and beta-TCP/Mg were significantly superior in bone formation than in control defects (
p < 0.05,
Table 3,
Figure 9).
Figure 9.
Micro-CT new bone area/tissue area formation, 40×. *
p
≤ 0.05, **
p
≤ 0.01, and ****
p
≤ 0.0001.
Table 3. Bone formation by Microcomputed Tomography scan (μCT; %).
| |
Week Two |
Week Six |
Week Eight |
| β-TCP/Mg |
22.73 ± 5.39 |
28.36 ± 1.70 |
32.65 ± 1.24 |
| β-TCP |
21.74 ± 4.24 |
24.73 ± 4.41 |
28.98 ± 2.65 |
| Control |
14.54 ± 5.23 |
15.58 ± 5.45 |
24.1 ± 4.05 |
Up to 28.36% ± 1.70% new bone regeneration was achieved by β-TCP/Mg during week 6, which was significantly higher than that observed for the β-TCP control (24.73% ± 4.41%) and control groups (15.58% ± 5.45%). Statistically significant differences were exhibited between both the beta-TCP/Mg and beta-TCP (
p < 0.01) with β-TCP/Mg and the control group
(p < 0.05) (
Table 3,
Figure 9).
At eight weeks, β-TCP/Mg induced 32.65% ± 1.24% bone regeneration. In addition, new bone formation was significantly more extensive in the β-TCP/Mg group than it was in the β-TCP control (28.98% ± 2.65%) or in the control group (24.1% ± 4.05%) (
p < 0.05,
Table 3,
Figure 9).
Additionally, control defects did not close and maintained the space that they took up. In contrast, the β-TCP/Mg and untreated β-TCP particles managed to yield greater defect closure and space maintenance at eight weeks (
Table 3,
Figure 9).
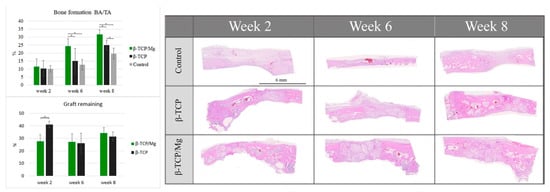
4. In Vivo Biocompatibility
The control group regenerated 10.14% ± 2.11% new bone, which did not differ significantly from the 10.42% ± 4.89% of the β-TCP control group and the 11.48% ± 4.88% of the β-TCP/Mg group (
p < 0.05) (
Table 4,
Figure 10). β-TCP/Mg had faster graft degradation, with 27.73 ± 12.50% graft remaining, while the β-TCP control had a lower graft resorption rate with 41.10 ± 6.80% (
p < 0.05) (
Figure 10).
Figure 10.
Histologic BA/TA new bone formation and remaining graft 40×. *
p
≤ 0.05.
Table 4. Histology of newly formed bone (%).
| |
Week Two |
Week Four |
Week Eight |
| β-TCP/Mg |
11.48 ± 4.88 |
24.42 ± 4.53 |
31.62 ± 3.03 |
| β-TCP |
10.42 ± 4.89 |
15.07 ± 7.91 |
24.94 ± 3.00 |
| Control |
10.14 ± 2.11 |
12.73 ± 3.26 |
19.73 ± 3.37 |
On week 6, more additional new bone was found within the particles. The β-TCP control generated 15.07 ± 7.91% more new bone than the control group did: 12.73% ± 3.26%. Additionally, β-TCP/Mg regenerated 24.42% ± 4.53% new bone, which was greater than that of the control (
p < 0.001) and β-TCP control (
p < 0.05) defects (
Table 4,
Figure 10). Both β-TCP/Mg and β-TCP had similar graft resorption rates with 27.30 ± 14.84% and 26.20 ± 20.1%, respectively.
On week 8, the graft particles started reabsorbing while regenerating new bone formation, and bone maturation was able to be seen through defects because of the particle scaffold characteristics. The β-TCP/Mg (31.62% ± 3.03%) had better bone regeneration over the β-TCP control (
p < 0.05) and the control (
p < 0.001). Moreover, the β-TCP control group (24.94% ± 3.00%) had significantly higher amounts of new bone tissue than the control group (19.73% ± 3.37%,
p < 0.05), as shown in
Table 4 as well as in
Figure 10. The graft resorption rate had a similar tendency to the one observed at week 6, with both grafts having no statistically significant differences, with β-TCP/Mg and β-TCP show rates of 34.25 ± 3.40% and 31.57 ± 3.94%, respectively.
For the critical defects that were treated with β-TCP/Mg (histologic new bone formation: 31.62% ± 3.03%; micro-CT new bone formation: 32.65 ± 1.24), the new bone area was 4% greater on average than those treated with the β-TCP control (histologic new bone formation: 24.94% ± 3.00%; micro-CT new bone formation: 28.98 ± 2.65) (p < 0.05, Table 3 and Table 4).