1. Octreotide
Assuming that CC is caused by the massive release of hormones by tumor masses, octreotide has historically represented the mainstay of its treatment. This drug is a long-acting synthetic octapeptide
[1][34] that acts like somatostatin, a potent inhibitory peptide (
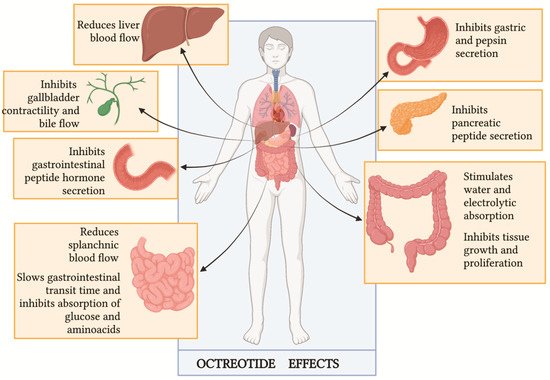
Figure 13). The blockade of hormone releases, such as insulin, glucagon, gastrin and other gastrointestinal molecules, and the reduction of splanchnic and hepatic blood flow represent the mechanisms underlying the management of CC. However, the real role of octreotide in the management of CC is not well established yet, and the available data are partly contradictory.
Figure 13. Octreotide clinical effects (Figure adapted by Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide
[2][35]).
The first report of using octreotide to treat CC dates back to 1985
[3][36], when Kvols et al. administered two intravenous boluses of 50 μg of this drug to a patient affected by a small bowel NET presenting life-threatening hypotension and prolonged flushing during abdominal surgery, unresponsive to intravenous fluids, intravenous calcium or intravenous conventional vasopressors. The Authors described a rapid resolution of the critical status and concluded that octreotide must be available in the operating room during surgery to rapidly manage CC. Furthermore, the same Authors conducted an explorative study on 25 patients with metastatic NET and documented CS
[4][37] with the aim to evaluate the effect of this long-acting somatostatin analog, suggesting that this drug could be routinely safely used in the management of CS with excellent results.
However, further studies have questioned the real role of octreotide in the specific treatment of CC. Considering the pharmacodynamic profile of octreotide, this drug acts as a hormone release blocker and not as a hormone receptor blocker, therefore it should not neutralize the effect of circulating vasoactive peptides
[5][6][38,39]. It should possibly prevent a worsening in the CC or prevent its occurrence at all. According to the most recent analysis put forward by Wann et al.
[7][40], the rapid resolution of the acute episode described by Kvols et al. could also be explained by a delayed effect of epinephrine or other previously administered medications.
In 2001, Kinney et al.
[8][9] evaluated the complication rate and outcomes of a larger series of patients with metastatic NET. Among the 119 subjects undergoing abdominal surgery included, 6 received only a preoperative octreotide dose, while 45 patients received octreotide intraoperatively, and 73 patients did not receive octreotide. A total of 15 out of 119 patients experienced perioperative complications, including 3 deaths, but none of the 45 subjects who received the intraoperative drug dose had an intraoperative complication. The researchers reported a statistically significant difference in terms of intraoperative complications between the 45 patients who received intraoperative octreotide and the 73 who did not (
p = 0.023). They concluded that patients with metastatic carcinoid tumors can undergo abdominal surgery safely with an intraoperative octreotide dose, reporting a significantly global decrease in intraoperative complications such as CC.
Based on the possible preventive role of octreotide on CC, Massimino et al. retrospectively explored the use of octreotide prophylaxis in a group of 97 patients undergoing surgery during the years 2007–2011. A total of 87 patients (90%) received prophylactic octreotide (dose range 100–1100 mg, median 500 mg), and 56% received at least one additional intraoperative dose. Despite the use of octreotide, intraoperative complications occurred in 23 (24%) patients. Therefore, the obtained data greatly diverged from the results published by Kinney et al., In their series, 18 patients (19%) experienced prolonged hypotension, while 5 (5%) were reported to have had marked hemodynamic instability consistent with a CC. Intraoperative complications occurred with the same frequency among patients with functioning (21%) and non-functioning (28%) NET, and the presence of liver metastases was found to be a predictor of intraoperative complications. These findings suggest neither outpatient octreotide LAR nor single-dose preoperative bolus octreotide prevent all intraoperative complications
[9][10].
In 2016, Woltering et al.
[10][41] suggested a possible solution to improve octreotide effectiveness. Their retrospective report demonstrated a reduction in CC incidence by using a continuous infusion of high-dose octreotide during surgery. As anesthetic or surgical stimuli can potentially precipitate an unpredictable release of amines, in their protocols, the researchers administered a prophylactic preoperative 500 μg bolus of octreotide acetate along with a continuous intravenous intraoperative infusion for all NET patients undergoing surgical cytoreduction, regardless of the location of their primary tumor or their functional status. The rationale behind this choice is connected to octreotide pharmacokinetics: preoperative bolus of octreotide, with a half-life of 90–120 min, might not last long enough for protection against CC during long surgery. Without a continuous infusion, blood level would fall to 50% of the original octreotide concentration after 2 h and would be only 25% of the original concentration after 4 h. As result, Woltering et al. reported an incidence of CC of only 3.6%, concluding that continuous intraoperative octreotide infusion could significantly reduce the risk of CC onset.
To demonstrate the benefits of continuous octreotide infusion on CC prevention, Condron et al.
[11] prospectively enrolled 127 patients (71% with liver metastases, 74% with CS) who underwent 150 operations with continuous octreotide infusions. Contrary to what was expected, 30% of the patients manifested CC, and the crises were significantly associated with the presence of liver metastases (
p = 0.02) or a history of CS (
p = 0.006). The rapid use of vasopressor was effective in reducing crisis duration, with a reduction in postoperative complications. These unexpected results could be explained by analyzing CC definitions. While Woltering et al. considered only episodes of hypotension lasting ≥10 min
[10][41], Condron et al. registered all cases of hemodynamic instability (systolic blood pressure < 80 or >180 mmHg, heart rate > 120 beats per minute or display of any physiology that, if sustained, would be expected to lead to end-organ dysfunction, such as ventricular arrhythmias or bronchospasm) unattributable to any other causes
[11]. Considering only episodes of hypotension lasting ≥10 min, as Woltering et al. did, Condron et al. would have reported only 8% of CC.
Other reports on the outcome of prophylactic octreotide were published in 2018
[12][42] and 2019
[13][12]. Kinney et al. retrospectively evaluated 169 patients undergoing partial hepatic resection for metastatic NET between 1997 and 2015, and 77% (130/169) of patients preoperatively received 500 μg of subcutaneous octreotide. In their report, there were no documented cases of CC; one patient developed clinical findings of an emerging CC but was successfully treated with doses of octreotide, and findings resolved in <10 min. Of note, in this case, CC was defined as a sudden or blunt onset of at least two of the following: flushing or urticaria that are not explained by an allergic reaction; bronchospasm or bronchodilator administration; hypotension (SBP < 80 mmHg for >10 min and treated with vasopressor) not explained by volume status or hemorrhage; tachycardia ≥120 beats per minute
[12][42]. Analyzing only sustained hypotension, the incidence was 5.6%, but because none of those patients exhibited any other criteria, none were considered CC.
Finally, Kwon et al. reported a retrospective series of 75 patients with metastatic well-differentiated NETs who underwent liver resection, ablation or embolotherapy from 2012 to 2016. The CC was defined subjectively by clinical documentation of occurrence by any treating physician, including the anesthesiologist, surgeon or interventional radiologist, and it had to be associated with hemodynamic instability, defined as the presence of at least one of the following events sustained for more than 10 min during the procedure: hypotension (systolic blood pressure, 80 mm Hg) or tachycardia (heart rate, >120 beats per minute). CC was identified in 32% (24) of patients. Of note, the route and dose of preprocedural octreotide administration varied widely. Neither long-acting octreotide, perioperative octreotide, intraoperative octreotide nor any combination was associated with a lower incidence of crisis. The Authors concluded that somatostatin analogs do not reliably prevent CC. One hypothesis is that CC may be a phenomenon physiologically distinct from CS, involving the release of a different distribution of vasoactive substances, against which different therapeutic agents can be used
[13][12]. As previously mentioned, this proposal has been explored by Condron et al.
[14][26].
Data are summarized in Table 13.
Table 13. Articles assessing the impact of octreotide in CC.
| Variation |
Type of Paper |
Number of Patients |
Number of CC |
Octreotide Dose and Regimen |
| Kvols et al., 1986 [3] |
Case report-retrospective study |
25 |
1 |
a bolus of 50 μg of octreotide intraoperatively |
| Kinney et al. [8] |
Retrospective study |
119 |
15 (none of the pts received onctreotide intraoperatively) |
- -
-
31 pts received octreotide preoperatively (median dose 300 μg—range 50–1000 μg); 25 of these pts received additional octreotide intraoperatively.
- -
-
45 pts received octreotide intraoperatively (median dose 350 μg—range 30–4000 μg)
|
| Massimino et al. [9] |
Retrospective study |
97 |
23 |
87 pts received prophylactic octreotide (median dose 500 μg—range 100–1100 μg) + intraoperative bolus if necessary (median dose 350 μg—range 100–5500 μg) |
| Woltering et al. [10] |
Retrospective study |
150 |
6 |
Continuous high-dose octreotide infusion: 500 μg/h |
| Condron et al. [11] |
Prospective study |
127 |
38 |
Continuous high-dose octreotide infusion: 100 μg/h |
| Kinney et al. [12] |
Retrospective study |
169 |
0 |
- -
-
130 pts received 500 μg preoperatively s.c.
- -
-
39 pts received additional intravenous octreotide (median dose 500 μg—range 250–650 μg)
|
| Kwon et al. [13] |
Retrospective study |
75 |
24 |
- -
-
27 pts received preprocedure infusion (median dose 150 μg/h—range 50–300 μg/h)
- -
-
21 pts received a preprocedure i.v. or s.c. bolus (median dose 150 μg—range 100–300 μg).
- -
-
48 pts received intraprocedural infusion (median dose 150 μg/h—range 50–300 μg/h)
- -
-
20 pts receive an intraprocedural i.v or s.c. bolus (median dose 150 μg—range 20–510 μg)
|
Articles assessing the impact of octreotide in CC.
As reported in the most recent guidelines referring to clinical studies discussed so far, there are no standard octreotide regimens in the management of CC; subcutaneous administration of octreotide 100–200 mcg × 2–3 daily during surgery has been suggested for a minor procedure or lower-risk patients. However, intravenous octreotide infusions should also be readily available in the operating room to be used when deemed necessary. For major surgery, perioperative prophylactic treatment with intravenous octreotide, at the starting dose of 50–100 mcg/h (mean dose 100–200 mcg/h), is the standard regimen used by most clinical centers. Although this has not been substantiated by any prospective study, most experts start treatment with intravenous octreotide 12 h before the operation and escalate the dose as necessary until symptom control is achieved. This infusion continues for at least 48 h after the operation, with dose titration as clinically required
[15][43].
Considering the increasing use of PRRT, several studies have been conducted to estimate the impact of octreotide in PRRT-induced CC. The incidence of CC during PRRT ranges between 1 and 10%, and, as previously mentioned in this
revie
ntryw, specific risk factors have been defined, which, if present, expose the patient to a major rate of intra-treatment complications
[16][24]. In the clinical experience reported by de Kaizer et al., among 479 patients enrolled in the study, 7 cases of CC after the first cycle of PRRT were reported
[17][44]. The treatment included high-dose octreotide, i.v. fluid replacement and corticosteroid. Despite additional precautions taken after their first therapy cycle (continuation of somatostatin analog, corticosteroids and reduction of administered dosage of
177Lu-octreotate), 3 patients developed a second CC after the subsequent cycle of PRRT. Analyzing the possible mechanism behind hormonal secretion during Lu-octreotate therapy (tumor lysis vs. discontinuation of short-acting somatostatin analog vs. emotional stress response to hospitalization vs. administration of arginine and lysine), the Authors concluded that hormonal crises should be managed with an infusion of somatostatin analogs i.v., fluids, corticosteroids and correction of electrolyte disturbances. More recently, Stenzel et al. came to the same conclusions
[18][45]. Moreover, the Australian group of Tapia Rico et al. first proposed a protocol to prevent and manage severe CC and specifically defined which patients would benefit most (“high-risk patients”) from premedication to PRRT therapy with corticosteroids and bolus dose of octreotide sc
[19][46]. This work has been further enriched by De Olmo et al., who recently published the current procedure adopted for approaching patients undergoing PRRT
[16][24].
The work of clinicians should start from the identification of risk factors for CC through:The work of clinicians should start from the identification of risk factors for CC through:
The evaluation of nutritional assessment with the diagnosis and correction of hydro electrolytic disorders, malnutrition and malabsorption, and the avoidance of food triggers and intensive physical exercises the previous day;
- -
-
The evaluation of nutritional assessment with the diagnosis and correction of hydro electrolytic disorders, malnutrition and malabsorption, and the avoidance of food triggers and intensive physical exercises the previous day;-
- -
-
The evaluation of NET characteristics (high tumor burden, use of somatostatin analogs to control CS).-
The evaluation of NET characteristics (high tumor burden, use of somatostatin analogs to control CS).
In particular, in the case of bulky tumors, it is mandatory to consider a possible surgery or locoregional treatment before PRRT. In addition, it is essential to obtain good control of CS before the first cycle of 177Lu-octreotate.
In particular, in the case of bulky tumors, it is mandatory to consider a possible surgery or locoregional treatment before PRRT. In addition, it is essential to obtain good control of CS before the first cycle of 177Lu-octreotate.
As premedication, the Authors advise the administration of corticosteroids (dexamentasone 4–8 mg), antiemetic (ondansetron 4 mg), somatostatin analog (octreotide 100 μg s.c. or 50 μg i.v. bolus), antistaminic h1 (dexchlorpheniramine 5 mg i.v. in slow infusion) and antistaminic H2 (ranitidine 50 mg i.v. in slow infusion). In the event of an outbreak of CC, the infusion of Lu-DOTATATE should immediately stop, and a bolus of octreotide (100–500 μg) should be immediately administered, followed by a maintaining dose of 50–100 μg/h infusion. In case of severe hypotension, the Authors do not exclude the use of phenylephrine or vasopressor drugs.