1. Evolution
The description of polycystic ovary syndrome (PCOS) phenotypes can be found in medical records from antiquity and the modern syndrome was described over 80 years ago
[1][2][17,59]. Nevertheless, there is ongoing debate regarding the evolutionary origins of PCOS
[3][4][1][5][6][7][8][9][10][15,16,17,39,60,61,62,63,64]. PCOS susceptibility alleles may have arisen in
theour phylogenetic ancestors, in the hunter–gatherer Paleolithic period of the Stone Age, after the Neolithic Agricultural Revolution or following the Industrial Revolution
[4][1][16,17]. From an evolutionary perspective, nearly all genetic variants that influence disease risk have human-specific origins, but the systems they relate to have ancient roots in
theour evolutionary ancestors
[11][8]. Regardless of the precise timing of the origin of PCOS in humans, the complex metabolic and reproductive gene variants identified in women with PCOS relate to ancient evolutionary-conserved metabolic and reproductive survival pathways
[3][12][15,29]. Although evolutionary hypotheses about disease vulnerability are impossible to prove they have the potential to frame medical thinking and direct scientific research for the proximate causes of disease
[3][6][15,60].
Multiple hypotheses have been proposed regarding the evolutionary origins of PCOS and related metabolic diseases
[11][6][9][8,60,63]. These hypotheses are focused on the relative importance of metabolic survival adaptations versus improved reproductive success, or a combination of both. A detailed analysis of these hypotheses, and the complexities of the evolutionary considerations
, ha
sve been
updatedreviewed elsewhere and is beyond the scope of the present review [11][6][8,60]. One common theme is that PCOS may be viewed as a “conditional phenotype” where a specific set of conditions has unmasked normally unexpressed or partly expressed genetic pathways, which then provide a survival advantage under certain environmental conditions
[13][4][14,16].
All organisms have physiological adaptive responses to deal with changing environmental conditions (starvation, fasting, physical threat, stress and infection) and the varying demands of internal physiological states (pregnancy, lactation and adolescence)
[13][14][14,65]. It has been proposed that the PCOS phenotype may have been invoked in specific environmental conditions in ancestral populations as a short, medium or even long-term adaptive survival mechanism
[3][4][1][15,16,17]. The view of PCOS as a conditional phenotype proposes that these physiological responses become pathological in
theour modern environment due to factors such as food abundance, reduced physical activity, circadian disruption, stress and environmental chemical exposure. The transgenerational evolutionary theory of the pathogenesis of PCOS encompasses all of the above ideas to explain the observed pathophysiological and clinical features of PCOS
[15][28].
It is generally accepted that almost all pre-industrial societies and animal populations experienced seasonal or unpredictable episodes of food shortage that applied evolutionary pressure to develop metabolic and reproductive adaptive survival responses
[1][16][17,49]. It is also appreciated that metabolic and reproductive pathways are interconnected and involve reciprocal feedback control mechanisms
[17][18][19][66,67,68]. During periods of starvation, anorexia or excessive weight gain, reproduction is down-regulated and ovulation becomes irregular or ceases
[20][21][69,70]. Similarly, metabolic function is coordinated with the menstrual cycle to ensure optimal physiological conditions for fertilization, implantation, pregnancy, parturition and lactation
[22][71]. Recent research has elaborated on the details of how some of these complex regulatory mechanisms interact using specific hormonal, nutrient sensing and intracellular signaling networks
[23][24][25][72,73,74].
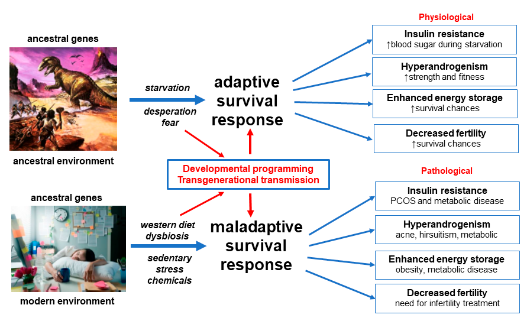
Details of the mechanisms underlying the proposed adaptive survival advantages of IR, hyperandrogenism, enhanced energy storage and subfertility have been obtained from paleolithic records, animal models and human populations exposed to adverse environmental conditions such as war and famine-inflicted starvation
[13][4][8][9][14,16,62,63]. Multiple lines of evidence support the maladaptive response of human populations to rapidly changing nutritional, physical, psychological and cultural environments, in the modern world
[26][27][13][28][5,11,14,75]. These “adaptations” result in pathological responses to IR, hyperandrogenism, enhanced energy storage and ovulation
(Figure 1).
Figure 1. Evolutionary model of the pathogenesis of polycystic ovary syndrome. Reprinted from Ref. [12].
Theories of evolutionary mismatch have also been advanced to explain all of the cluster of metabolic diseases associated with PCOS (type 2 diabetes, metabolic syndrome,
non-alcoholic fatty liver disease (NAFLD
) and cardiovascular disease) and follow the same set of basic principles and explanations
[13][30][14,76]. This common body of evolutionary evidence is supported by the increasing incidence of metabolic-related disease, such as diabetes and obesity, in developed countries and in developing nations adopting a Western diet and lifestyle
[27][31][11,77]. In addition, the demonstrated reversibility of PCOS and related metabolic and biochemical features following changes in diet, increased physical activity and other lifestyle interventions, adds further support to a transgenerational evolutionary model
[32][33][50,51].
2. Genetics
The heritable nature of PCOS has been proposed since the 1960′s following a range of familial, twin and chromosomal studies
[34][35][36][78,79,80]. Cytogenetic studies failed to identify karyotypic abnormalities and genetic studies did not show a monogenic inheritance pattern following examination of candidate genes
[37][38][81,82]. In addition, two or more phenotypes can be present in the same family suggesting that some of the phenotypic differences could be accounted for by variable expression of the same shared genes
[37][39][81,83].
The mapping of the human genome in 2003
[40][84] and the publication of the human haplotype map (more than one million single nucleotide polymorphisms of common genetic variants) in 2005
[41][85], lead to the realization that most DNA variation is shared by all humans and is inherited as blocks of linked genes (linkage disequilibrium)
[42][86]. These advances enabled a revolution in case-control studies and the development of Genome-Wide Association Studies (GWAS) which map the entire human genome looking for susceptibility genes for complex traits such as obesity, type 2 diabetes and PCOS
[37][81].
The first PCOS GWAS was published in 2010 and demonstrated 11 gene loci associated with PCOS
[43][87]. Additional loci have subsequently been found in several different ethnic groups
[42][44][86,88]. The first GWAS analysis of quantitative traits was published in 2015 and showed that a variant (rs11031006) was associated with luteinizing hormone levels
[44][88]. The largest GWAS included a meta-analysis of 10,074 PCOS cases and 103,164 controls and identified 19 loci that confer risk for PCOS
[12][29]. The genes associated with these loci involve gonadotrophin action, ovarian steroidogenesis, insulin resistance and type 2 diabetes susceptibility genes. The first GWAS using electronic health record-linked biobanks has introduced greater investigative power and identified 2 additional loci
[45][89]. These variants were associated with polycystic ovaries and hyperandrogenism (rs17186366 near
SOD2) and oligomenorrhoea and infertility (rs144248326 near
WWTR1)
[45][89]. In addition to identifying common gene variants for PCOS phenotypes, finding the same signals (THADA, YAP1 and c9orf3) in Chinese and European populations suggests that PCOS is an ancient trait that was present before humans migrated out of Africa
[37][81].
More recently Mendelian randomization (MR) studies have been used to explore the potential causative association between gene variants identified in
(GWAS
) and PCOS
[46][47][90,91]. Many of the gene variants identified in GWAS are located in non-coding regions of DNA
[48][92]. The genes or functional DNA elements through which these variants exert their effects are often unknown. Mendelian randomization is a statistical methodology used to jointly analyze GWAS and quantitative gene loci to test for association between gene expression and a trait, due to a shared or potentially causal variant at a specific locus
[49][93]. A detailed analysis of MR methodology and the limitations of this statistical tool is beyond the scope
of the present review. Although MR studies have the potential to infer causation it is recognized that they also have limitations in PCOS research
[46][90]. Nevertheless, preliminary evidence suggests that several genes related to obesity, metabolic and reproductive function, may play a causal role in the pathogenesis of PCOS
[46][47][90,91].
Decades of genetic research has therefore characterized PCOS as a polygenic trait that results from interactions between the environment and susceptible genomic traits
[50][12][35][44][27,29,79,88]. The failure to identify a qualitative or monogenic inheritance pattern and the findings from GWAS, MR, familial and twin studies, suggests that the heritability of PCOS is likely to be due to the combination of multiple genes with small effect size, as has been found with obesity and type 2 diabetes
[35][36][51][52][53][79,80,94,95,96]. Polygenic traits are the result of gene variants that represent one end of the bell-shaped normal distribution curve of continuous variation in a population
[54][97]. From an evolutionary perspective, women with PCOS may represent the “metabolic elite” end of the normal distribution curve, being able to efficiently store energy in periods of food abundance and down-regulate fertility in times of food scarcity, or even in anticipation of reduced seasonal food availability as a predictive adaptive response
[4][1][6][16,17,60].
The realization that PCOS is a quantitative trait (phenotype determined by multiple genes and environmental factors) has far-reaching implications for the diagnosis, treatment and prevention of symptoms and pathology associated with PCOS. The implications require a shift in thinking about PCOS as a “disease” to a variation of normal metabolic and reproductive function. This shift invites a change in vocabulary from talking about “disorder” and “risk” to talking about “expression” and “variability”
[54][97]. This new understanding supports and reinforces an evolutionary model of the pathogenesis of PCOS. In keeping with this model, multiple lines of evidence suggest that inherited PCOS gene variants are developmentally programmed in a way that primes them for activation by nutritional and environmental factors in postnatal life
[55][56][57][41,42,98].
3. Developmental Epigenetic Programming
The developmental programming of PCOS represents changes in gene expression that occur during critical periods of fetal development
[58][99]. Following fertilization, most parental epigenetic programming is erased and dramatic epigenomic reprogramming occurs
[59][100]. This results in transformation of the parental epigenome to the zygote epigenome and determines personalized gene function. Compelling evidence shows that a wide range of maternal, nutritional and environmental factors can effect fetal development during these critical periods of programming
[60][57][58][61][62][44,98,99,101,102]. These include hormones, vitamins, diet-derived metabolites and environmental chemicals
[63][57][64][65][48,98,103,104]. In addition, epigenetic reprogramming of germ-line cells can lead to transgenerational inheritance resulting in phenotypic variation or pathology in the absence of continued direct exposure
[57][98].
Experimental studies in primates, sheep, rats and mice show that PCOS-like syndromes can be induced by a range of treatments including androgens, anti-Mullerian hormone and letrozole
[66][60][67][19,44,46]. Nevertheless, there is significant debate regarding when an animal model qualifies as PCOS-like
[68][105]. The model used and the method of induction of PCOS phenotypes therefore needs to be carefully scrutinized when generalizing findings from animal research to women with PCOS. Most of the animal and human research on the developmental origins of PCOS has focused on the role of prenatal androgen exposure. This
whas
i been extensively reviewed in numerous previous publications
[55][67][41,46]. This research has resulted in a proposed “two hit” hypothesis for the development of PCOS phenotypes
[69][70][43,45]. The “first hit” involves developmental programming of inherited susceptibility genes and the “second hit” arises due to lifestyle and environmental influences in childhood, adolescence and adulthood
[55][71][41,106].
If PCOS is a quantitative trait involving normal gene variants, as suggested by the evolutionary considerations and findings from genetic research, then the “first hit” may result from normal developmental programming events as occurs with other gene variants
[62][102]. According to this hypothesis, the polygenic susceptibility genes would be normally “activated” and “primed” to respond to future maternal and environmental conditions and exposures, as would be the case with many other normal genes
[15][28]. In addition, the susceptibility alleles may be “activated” or “functionally enhanced” by a range of maternal and environmental factors, as is usually presumed to be the case in PCOS
[26][13][62][5,14,102]. This developmental plasticity would provide a mechanism for a predictive adaptive response, based on inputs from the maternal environment that could be used to program metabolic and reproductive survival pathways, to better prepare the offspring for the future world in which they may be expected to live
[72][107].
Parental lifestyle factors including diet, obesity, smoking and endocrine-disrupting chemicals, have all been shown to modulate disease risk later in life
[65][73][74][104,108,109]. The original description of the fetal origin’s hypothesis proposed that poor maternal nutrition would increase fetal susceptibility to the effects of a Western-style diet later in life
[16][49]. Subsequent studies have confirmed that maternal exposure to either nutrient excess or deficit, can have long-term consequences for the health of the progeny
[65][104]. Evidence from human and animal studies suggests that maternal obesity programs the offspring for increased risk of developing obesity, hyperglycemia, diabetes, hypertension and metabolic syndrome
[73][108].
The developmental origins of PCOS may have been due to different factors in ancestral and modern populations
[1][6][17,60]. It has been hypothesized that environmental stress, infection, nutrient deprivation, fetal growth restriction and stress hormone responses may have resulted in maternally mediated modulation of gene expression in ancestral offspring
[1][75][17,110]. Some of these factors have been investigated and confirmed in modern populations subject to starvation and extreme environmental conditions
[76][111]. In contrast, altered fetal programming in modern societies may be secondary to maternal overnutrition, sedentary behavior, obesity, emotional stress, circadian rhythm disruption, poor gastrointestinal health or environmental chemical exposure
[77][61][78][79][35,101,112,113]. The preconception and pregnancy periods therefore provide a unique opportunity for lifestyle interventions that promote optimal future health for both the mother and the offspring
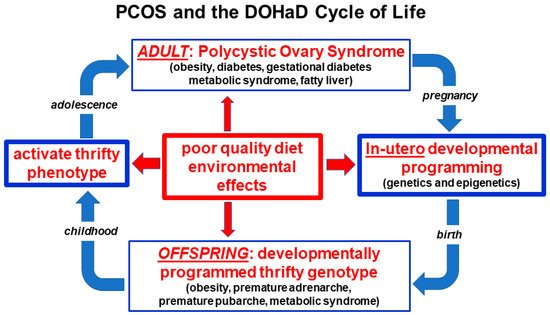
(Figure 2).
Figure 2. Nutritional and environmental influences throughout the life course and the perpetuation of the transgenerational inheritance of polycystic ovary syndrome. Reprinted from Ref. [28].
4. Microbiome and Dysbiosis
The gastrointestinal microbiome is now appreciated to play a central role in human health and disease
[80][81][114,115]. The microbiome is known to co-regulate many physiological functions involving the immune, neuroendocrine and metabolic systems via complex reciprocal feedback mechanisms that operate between the microbial ecosystem and the host
[82][83][116,117]. Evidence from studies in Western populations, hunter–gatherer societies and phylogenetic studies in other species, have attempted to place the human microbiome into an evolutionary context
[84][118]. Although microbes clearly impact host physiology and have changed along branches of the evolutionary tree, there is ongoing debate regarding whether the microbiome can evolve according to the usual evolutionary forces
[85][86][119,120]. Nevertheless, it has been argued that focusing on functional pathways and metabolic roles of microbial communities, rather than on specific microbes, provides a better model for understanding evolutionary fitness
[84][118]. The co-evolution of the microbiome and human physiology may therefore be important in understanding the differences between ancient adaptive physiological survival mechanisms and modern lifestyle-related pathological responses, in women with PCOS
(Figure 1).
Twin studies and GWAS show that host genetics can influence the microbiome composition, and microbes can exert effects on the host genome, although the environment has an important role
[87][88][121,122]. Gastrointestinal and immune cells are constantly interacting with the microbiome to try to determine which microorganisms are beneficial or harmful. Immune genes involved in this process are the most rapidly evolving protein-encoding genes in the mammalian genome
[89][90][123,124]. Diversification of microbes allows humans to access dietary niches and nutritional components they otherwise would not be able to access, which may be beneficial and ultimately lead to the integration of specific microbes into the ecosystem
[91][125]. Although no living population today carries an ancestral microbiome, comparison studies of non-Western and Western populations show significant differences in the relative abundances of common phyla and a much greater species diversity in non-Western populations
[92][93][126,127].
A There isreview of non-human primate and human gut microbiome datasets, revealed a changing microbiome in response to host habitat, season and diet, although there appear to be common species-specific symbiotic communities
[84][118].
Rapid human cultural changes have resulted in significant dietary modifications in urban-industrialized communities and shifted the microbiome at an unprecedented rate. The result has been the development of a mismatch between human metabolic genes and bacteria that enhance fat storage
[94][128]. In
theour evolutionary past, when nutrients were scarce, it has been theorized that host selection led to the maintenance of microbes that enhance nutrient uptake or host energy storage. However, in the modern environment, where a high-fat, high-sugar, low-fiber diet has become common and easily accessible, integration of these microbes leads to maladaptive physiological responses
[95][40]. For metabolically thrifty individuals with PCOS, harboring microbes that enhance energy storage escalates the evolutionary conflict, furthering the development of insulin resistance and therefore progression to obesity and type 2 diabetes
[29][96][12,129]. Further compounding this maladaptive response is the loss of microbes that are required to access other dietary niches. One example is the loss of symbiotic species of Treponema in individuals living in urban-industrialized communities
[97][130]. A change from the ancestral hunter–gatherer diet, where foods consumed changed seasonally and a wide variety of food components were eaten, to a diet that is similar across seasons and significantly less varied, is another likely contributor to reduced diversity of the microbiomes of individuals living in urbanized–industrialized communities
[98][131].
The majority of women with PCOS are overweight or obese and evidence indicates that the microbiome of obese individuals is capable of extracting more energy from the host diet compared with the microbiome of lean individuals
[99][132]. This is thought to be driven by an expansion in pro-inflammatory species of bacteria, such as
E. coli, and a depletion of anti-inflammatory bacteria such as
Faecalibacterium prausnitzii [100][101][133,134]. Chronic low-grade ‘metabolic’ inflammation, or meta-inflammation, is a result of an imbalanced gastrointestinal microbiota that promotes the development of insulin resistance and type 2 diabetes
[102][103][104][135,136,137].
The dysbiosis of gut microbiota theory of PCOS, proposed by Tremellen in 2012, accounts for the development of all of the components of PCOS (multiple ovarian follicles, anovulation or menstrual irregularity and hyperandrogenism)
[95][40]. The theory proposes that a poor-quality diet and resulting imbalanced microbiome, induces intestinal permeability and endotoxemia, exacerbating hyperinsulinemia. Increased insulin levels promote higher androgen production by the ovaries and disrupts normal follicle development. Metabolic, endocrine and environmental factors associated with PCOS are not mutually exclusive, and therefore their relative contributions to dysbiosis in PCOS remains uncertain
[105][138]. Consuming a balanced diet that is low in fat and high in fiber, can also restore balance to the ecosystem (termed eubiosis)
[32][50]. A recent study showed that dietary intake of fiber and vitamin D was significantly decreased in both lean and obese women with PCOS, compared to healthy controls, and correlated with lower diversity of the gut microbiome
[106][139]. Dysbiosis is reversible with improvement in diet quality augmented by the addition of probiotics or synbiotics
[33][107][108][109][51,56,57,58].
Dysbiosis is a consistent finding when looking at the microbiome of women with PCOS
[110][111][112][113][140,141,142,143]. Although most studies are small, dysbiosis has consistently been found to correlate with different physiological parameters, such as obesity, sex hormones and metabolic defects
[110][111][113][140,141,143]. Similar to microbiomes associated with obesity, the microbiomes of individuals with PCOS have generally been found to have lower alpha diversity (lower numbers of bacterial taxa) than controls, and most studies describe an altered composition of taxa relative to controls
[110][113][140,143]. However, the bacterial taxa observed to be either increased, depleted or absent in PCOS differs from study to study. This is likely due to both the immense inter-individual variation in microbiotas, as well the fact that PCOS is a quantitative trait with women with various degrees and levels of obesity and sex hormones.
In keeping with the developmental origins hypothesis previously discussed, maternal androgens may alter the composition and function of the microbiome, therefore facilitating the pathogenesis of PCOS
[110][140]. One study showed that beta diversity, which is used to measure differences between groups, was negatively correlated with hyperandrogenism, suggesting that androgens play a significant role in dysbiosis
[110][140]. The ‘first hit’ in utero may therefore combine with vertical transmission of a dysbiotic microbiome from a mother with PCOS, resulting in dysbiosis in the offspring. Preconception and pregnancy provide a unique opportunities for lifestyle and dietary interventions aimed at restoring eubiosis, to enable the transference of a balanced ecosystem to the offspring, via vertical transmission
[84][118].
The accumulating scientific evidence strongly supports the significant role played by the microbiome in the pathogenesis and maintenance of PCOS, consistent with research in other related metabolic conditions. The role of dysbiosis is supported by over 30 proof-of-concept studies
that have [114]recently been reviewed [144]. Dysbiosis is therefore a significant factor in the pathogenesis of PCOS and an important component of a unified evolutionary model. Dysbiosis represents a maladaptive response of the microbiome to modern lifestyle influences and is a modifiable factor in the treatment of women with PCOS.
5. Insulin Resistance
There are several dilemmas when assessing the role of IR in women with PCOS. There is no consensus on the definition of IR
[115][116][145,146], measurement is difficult
[117][118][147,148], whole-body IR is usually measured although it is recognized that IR can be selective being either tissue-specific or pathway-specific within cells
[119][120][121][149,150,151], normal values are categorical and determined by arbitrary cut-offs (4.45 mg/kg/min)
[115][145], testing is not recommended in clinical practice
[122][38], reported prevalence rates in obese and lean women vary widely
[117][123][147,152], and the significance of IR as a pathognomonic component of PCOS is an area of debate
[124][125][126][153,154,155].
Despite these limitations, it is hypothesized that IR is a significant proximate cause of PCOS and is intrinsic to the underlying pathophysiology
[60][127][44,156]. In addition, it is recognized that IR plays a major role in the pathophysiology of all of the metabolic diseases, cardiovascular disease, some neurodegenerative diseases, and selected cancers
[128][129][22,157]. Insulin resistance is therefore considered to be the main driver for many diseases and makes a significant contribution to the chronic disease epidemic
[130][158]. Nevertheless, being able to vary the sensitivity and physiological action of insulin is thought to have conferred a significant adaptive survival role in many animals throughout evolutionary history
[116][131][146,159]. It has been proposed that IR may have evolved as a switch in reproductive and metabolic strategies, since the development of IR can result in anovulation and reduced fertility, in addition to differential energy repartitioning to specific tissues
[131][159].
Insulin receptors are located on the cell membranes of most tissues in the body
[132][160]. Ligand binding to the alpha-subunit induces autophosphorylation of specific tyrosine residues on the cytoplasmic side of the membrane
[132][133][160,161]. The activated insulin receptor initiates signal transduction via the phosphatidylinositol-3 kinase (PI-3K) metabolic pathway and the mitogen-activated protein kinase pathway (MAPK) which is involved in cell growth and proliferation
[133][161]. Insulin is an anabolic hormone that facilitates glucose removal from the blood, enhances fat storage and inhibits lipolysis in adipose tissue, stimulates glycogen synthesis in muscle and liver and inhibits hepatic glucose output
[133][161]. IR can be defined as a state where higher circulating insulin levels are necessary to achieve an integrated glucose-lowering response
[116][146]. IR results from alterations to cellular membrane insulin-receptor function or intracellular signaling, enzyme, metabolic or gene function
[116][132][133][146,160,161].
Insulin resistance can be caused by a wide variety of mechanisms that have the ability to disrupt any part of this metabolic signaling system
[134][133][53,161]. These include autoantibodies, receptor agonists and antagonists, hormones, inflammatory cytokines, oxidative stress, nutrient sensors and metabolic intermediates
[132][133][135][136][160,161,162,163]. Physiological regulation of insulin function can be viewed as an adaptive mechanism to regulate the metabolic pathway of insulin signaling (PI-3K), in response to changing environmental conditions [starvation, fear, stress]
[137][138][164,165] or during normal alterations of internal states (pregnancy, lactation, adolescence)
[14][116][123][65,146,152].
The physiological activation of IR allows the organism to switch from an anabolic energy storage state to a catabolic or energy mobilizing state. This allows free fatty acids to be mobilized from adipose tissue, which are then converted to glucose in the liver and released into the circulation
[133][161]. As a result of this metabolic change, blood sugar levels are maintained for vital metabolic processes and brain function
[13][14]. This adaptive protective mechanism can be pathway-specific during periods of growth, such as pregnancy, lactation and adolescence, so that only the metabolic signaling (PI-3K) is inhibited and not the mitogenic pathway (MAPK), which may even be up-regulated
[139][14][132][30,65,160].
When the physiology of insulin function is considered to be a quantitative or continuous variable from an evolutionary perspective, it is likely that all women with PCOS, whether obese or lean, have reduced insulin sensitivity
[123][126][140][152,155,166].
IA systemat
was fic review and meta-analysis of euglycemic-hyperinsulinemic clamp studies found that women with PCOS have a 27% reduction in insulin sensitivity compared to body mass index (BMI) and age-matched controls
[126][155]. In evolutionary terms, women with a PCOS metabolic phenotype would have increased survival chances during times of environmental or physiological demand for altered energy metabolism, but be more vulnerable to the pathological effects of IR when exposed to modern lifestyle factors
[13][1][131][14,17,159]. In particular, a poor-quality, high-glycemic, high-fat, low-fiber diet has been shown to cause IR
[95][141][40,167]. As discussed in the dysbiosis section, diet-related changes in the gastrointestinal microbiome have also been shown to cause IR in women with PCOS
[134][142][53,55]. Numerous studies have shown that dietary modification
[143][144][145][168,169,170], or treatment with prebiotics, probiotics or synbiotics, has the potential to restore normal insulin function
[108][146][57,171].
Consumption of a high-glycemic-load diet results in rapid increases in blood sugar levels that cause compensatory hyperinsulinemia
[141][147][167,172]. Excessive dietary intake of glucose and fructose are converted to fatty acids by de novo lipogenesis in the liver, transported to adipocytes via lipoproteins, released as fatty acids to adipocytes and stored in fat globules as triglycerides
[133][161]. As a result of nutrient overload, diacylglycerol, the penultimate molecule in the synthesis of triglyceride, accumulates in the cytoplasm and binds with the threonine amino acid in the 1160 position of the insulin receptor. This inhibits autophosphorylation and down-regulates the metabolic PI-3K pathway and causes IR
[133][161]. This process has the potential to be reversible following changes in diet quantity and quality, as has been shown to occur with calorie restriction, fasting, time-restricted eating, gastric bypass surgery, low saturated fat and low glycemic diets
[143][145][148][168,170,173]. Diets high in animal protein or saturated fat can also cause IR independent of BMI
[149][150][174,175]. These mechanisms provide the rationale for the principal recommendation of the International Guidelines that women with PCOS should be advised about dietary modification as the first line of management in all symptom presentations
[122][38].
6. Summary of the Unified Evolutionary Model
The evolutionary model proposes that PCOS is a condition that arises from the inheritance of genomic variants derived from the maternal and paternal genome. In utero fetal metabolic, endocrine and environmental factors modulate developmental programming of susceptible genes and predispose the offspring to develop PCOS. Postnatal exposure to poor-quality diet, sedentary behavior, EDC, circadian disruption and other lifestyle factors activate epigenetically programmed pathways, resulting in the observed features. Dietary factors cause gastrointestinal dysbiosis and systemic inflammation, insulin resistance and hyperandrogenism. Continued exposure to adverse lifestyle and environmental factors eventually leads to the development of associated metabolic conditions such as obesity, gestational diabetes (GDM)GDM, diabetes, NAFLD and metabolic syndrome (Figure 1).
Balanced evolutionary selection pressures result in transgenerational transmission of susceptible gene variants to PCOS offspring. Ongoing exposure to adverse nutritional and environmental factors activate developmentally programmed genes and ensure the perpetuation of the syndrome in subsequent generations. The DOHaD cycle can be interrupted at any point from pregnancy to birth, childhood, adolescence or adulthood by targeted intervention strategies (Figure 2).
In summary, PCOS is porposed thatwe propose that PCOS is an environmental mismatch disorder that manifests after in utero developmental programming of a cluster of normal gene variants. Postnatal exposure to adverse lifestyle and environmental conditions results in the observed metabolic and endocrine features. PCOS therefore represents a maladaptive response of ancient genetic survival mechanisms to modern lifestyle practices.