The natural human telomeric G-quadruplex (G4) sequence d(GGGTTAGGGTTAGGGTTAGGG) HT21 was extensively utilized as a G4 DNA-based catalytic system for enantioselective reactions. Modified oligonucleotides (ODNs) based on this sequence were investigated to evaluate their performances as DNA catalysts in an enantioselective sulfoxidation reaction of thioanisole. The HT21 derivative containing an AL residue in the first loop sequence significantly proved to be capable of producing about 84% enantiomeric excess.
- catalytic DNA
- telomeric G-quadruplex analogues
1. Introduction
| Name | Sequence (5′-3′) | Tm °C (±1) | ||
|---|---|---|---|---|
| HT21 | GGGTTAGGGTTAGGGTTAGGG | 69 | ||
| HT21-ABr1 | GGGTTA | Br | GGGTTAGGGTTAGGG | 70 |
| HT21-ABr2 | GGGTTAGGGTTA | Br | GGGTTAGGG | 72 |
| HT21-ABr3 | GGGTTAGGGTTAGGGTTA | Br | GGG | 71 |
| HT21-AL1 | GGGTTA | L | GGGTTAGGGTTAGGG | 73 |
| HT21-AL2 | GGGTTAGGGTTA | L | GGGTTAGGG | 74 |
| HT21-AL3 | GGGTTAGGGTTAGGGTTA | L | GGG | 68 |
| HT21-Aoxo1 | GGGTTA | oxo | GGGTTAGGGTTAGGG | 68 |
| HT21-Aoxo2 | GGGTTAGGGTTA | oxo | GGGTTAGGG | 68 |
| HT21-Aoxo3 | GGGTTAGGGTTAGGGTTA | oxo | GGG | 68 |
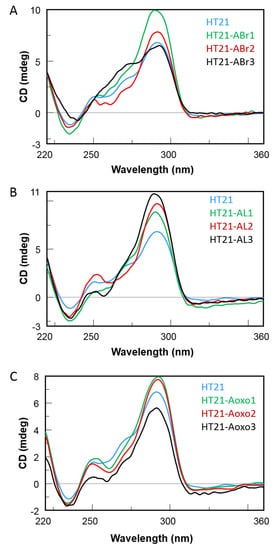
2. CD Spectroscopy
3. Catalytic Properties of HT21 Analogues
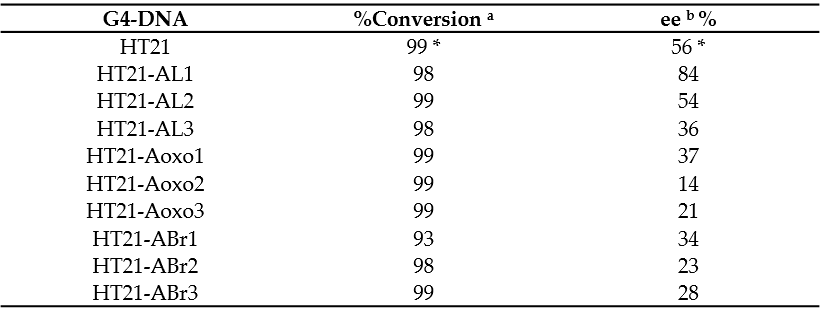
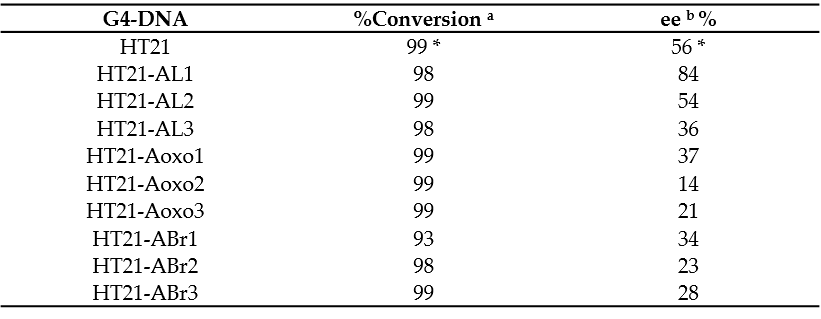
To investigate the catalytic properties of HT21 derivatives, the enantioselective sulfoxidation reaction, catalyzed by human telomeric G4 DNA and Cu(II) complexed with 4,4′-bimethyl-2,2′-bipyridine (CuL), using H2O2 as the oxidant, was carried out on thioanisole giving sulfoxide enantiomers. The reaction conditions used, including molar ratio between G4 DNA catalyst and CuL cofactor, potassium concentration, buffer pH, and temperature, were those optimized by Mingpan Cheng et al. [11]. In these conditions ([G4 DNA]/[CuL] = 1:5, [KCl] = 150 mM, pH 7.0, 15 °C), the unmodified telomeric G4 DNA catalyzed the reaction with a full conversion and 56% enantioselective excess (ee) [11].
All the modified HT21 catalysts, except HT21-ABr1 (93% of conversion), complexed with CuL, exhibited conversion data very similar to the natural one, thus indicating that, in general, the introduction of an unnatural adenosine in the HT21 loop sequence did not reduce the catalytic activities of the evaluated modified G4 DNA. However, data clearly indicated that the presence of modified residues significantly affected the enantioselectivities of the G4-based catalysts (Table 2). Indeed, most of HT21-modified sequences revealed minor enantioselectivities (range of 14–37% ee) compared to the natural one, except in the cases of HT21-AL1 and HT21-AL2. Interestingly, while HT21-AL2 proved able to give an enantiomeric excess (54%) strictly comparable to the unmodified sequence, HT21-AL1 exhibited a remarkably improved enantioselectivity (about 84% ee). This ee value was significantly much higher than that of natural G4 DNA catalyst and, to the best of ourthe knowledge, corresponded to the highest enantioselectivity for DNA based oxidation reaction obtained to date.
|
G4-DNA |
%Conversion a |
ee b % |
|
HT21 |
99 * |
56 * |
|
HT21-AL1 |
98 |
84 |
|
HT21-AL2 |
99 |
54 |
|
HT21-AL3 |
98 |
36 |
|
HT21-Aoxo1 |
99 |
37 |
|
HT21-Aoxo2 |
99 |
14 |
|
HT21-Aoxo3 |
99 |
21 |
|
HT21-ABr1 |
93 |
34 |
|
HT21-ABr2 |
98 |
23 |
|
HT21-ABr3 |
99 |
28 |
| G4-DNA | %Conversion ab | ee b % |
|---|---|---|
| HT21 | 99 * | 56 * |
| HT21-AL1 | 98 | 84 |
| HT21-AL2 | 99 | 54 |
| HT21-AL3 | 98 | 36 |
| HT21-Aoxo1 | 99 | 37 |
| HT21-Aoxo2 | 99 | 14 |
| HT21-Aoxo3 | 99 | 21 |
| HT21-ABr1 | 93 | 34 |
| HT21-ABr2 | 98 | 23 |
| HT21-ABr3 | 99 | 28 |
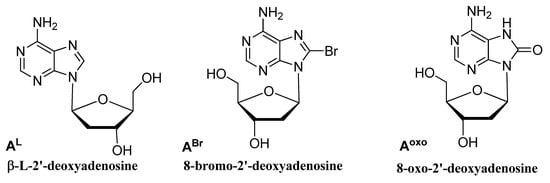
These data suggest that, in agreement with those reported for other reactions [9], the three-dimensional structure of HT21, generally preserved in all modified HT21 analogues as indicated by the close similarity of their CD spectra, ensured the notable catalytic performances, giving nearly quantitative conversion. On the other hand, the enantioselectivity of the product largely depended on the loop sequence of the G4 DNA catalyst. Minor modifications in the G4 DNA loop sequence, as the substitution of an A residue with an ABr or Aoxo, significantly reduced the chiral expression of sulfoxidation reaction. However, the replacement of a natural D-residue with a β-L-2′-deoxyadenosine in a specific loop was proven to be useful to increase the ee value of the reaction, thus improving chiral selectivity. OAurthors data confirmed the key role of the loop regions in close proximity to the terminal G-tetrads for chiral induction of the reactions and expanded the G4 DNA catalytic properties in asymmetric oxidations.
References
- Roelfes, G.; Feringa, B.L. DNA-based asymmetric catalysis. Angew. Chem.—Int. Ed. 2005, 44, 3230–3232.
- Coquière, D.; Feringa, B.L.; Roelfes, G. DNA-based catalytic enantioselective Michael reactions in water. Angew. Chem.—Int. Ed. 2007, 46, 9308–9311.
- Boersma, A.J.; Feringa, B.L.; Roelfes, G. Enantioselective friedel-crafts reactions in water using a DNA*based catalyst. Angew. Chem.—Int. Ed. 2009, 48, 3346–3348.
- Park, S.; Ikehata, K.; Watabe, R.; Hidaka, Y.; Rajendran, A.; Sugiyama, H. Deciphering DNA-based asymmetric catalysis through intramolecular friedel–crafts alkylations. Chem. Commun. 2012, 48, 10398–10400.
- Duchemin, N.; Heath-Apostolopoulos, I.; Smietana, M.; Arseniyadis, S. A decade of DNA-hybrid catalysis: From innovation to comprehension. Org. Biomol. Chem. 2017, 15, 7072–7087.
- Wang, J.; Benedetti, E.; Bethge, L.; Vonhoff, S.; Klussmann, S.; Vasseur, J.J.; Cossy, J.; Smietana, M.; Arseniyadis, S. DNA vs. mirror-image DNA: A universal approach to tune the absolute configuration in DNA-based asymmetric catalysis. Angew. Chem.—Int. Ed. 2013, 52, 11546–11549.
- Roe, S.; Ritson, D.J.; Garner, T.; Searle, M.; Moses, J.E. Tuneable DNA-based asymmetric catalysis using a G-quadruplex supramolecular assembly. Chem. Commun. 2010, 46, 4309–4311.
- Wang, C.; Jia, G.; Zhou, J.; Li, Y.; Liu, Y.; Lu, S.; Li, C. Enantioselective diels-alder reactions with G-quadruplex DNA-based catalysts. Angew. Chem.—Int. Ed. 2012, 51, 9352–9355.
- Wang, C.; Li, Y.; Jia, G.; Liu, Y.; Lu, S.; Li, C. Enantioselective friedel-crafts reactions in water catalyzed by a human telomeric G-quadruplex DNA metalloenzyme. Chem. Commun. 2012, 48, 6232–6234.
- Tang, Z.; Gonçalves, D.P.N.; Wieland, M.; Marx, A.; Hartig, J.S. Novel DNA catalysts based on G-quadruplex recognition. ChemBioChem 2008, 9, 1061–1064.
- Cheng, M.; Li, Y.; Zhou, J.; Jia, G.; Lu, S.M.; Yang, Y.; Li, C. Enantioselective sulfoxidation reaction catalyzed by a G-quadruplex DNA metalloenzyme. Chem. Commun. 2016, 52, 9644–9647.
- Burge, S.; Parkinson, G.N.; Hazel, P.; Todd, A.K.; Neidle, S. Quadruplex DNA: Sequence, topology and structure. Nucleic Acids Res. 2006, 34, 5402–5415.
- Dai, J.; Carver, M.; Yang, D. Polymorphism of human telomeric quadruplex structures. Biochimie 2008, 90, 1172–1183.
- Phan, A.T. Human telomeric G-quadruplex: Structures of DNA and RNA sequences. FEBS J. 2010, 277, 1107–1117.
- Dey, S.; Jäschke, A. Tuning the Stereoselectivity of a DNA-Catalyzed Michael Addition through Covalent Modification. Angew. Chem.—Int. Ed. 2015, 54, 11279–11282.
- Dey, S.; Jäschke, A. Covalently functionalized DNA duplexes and quadruplexes as hybrid catalysts in an enantioselective friedel-crafts reaction. Molecules 2020, 25, 3121.
- Wang, C.; Jia, G.; Li, Y.; Zhang, S.; Li, C. Na+/K+ switch of enantioselectivity in G-quadruplex DNA-based catalysis. Chem. Commun. 2013, 49, 11161–11163.
- Li, Y.; Cheng, M.; Hao, J.; Wang, C.; Jia, G.; Li, C. Terpyridine-Cu(II) targeting human telomeric DNA to produce highly stereospecific G-quadruplex DNA metalloenzyme. Chem. Sci. 2015, 6, 5578–5585.
- Cheng, M.; Hao, J.; Li, Y.; Cheng, Y.; Jia, G.; Zhou, J.; Li, C. Probing the interaction of copper cofactor and azachalcone substrate with G-quadruplex of DNA based Diels-Alderase by site-specific fluorescence quenching titration. Biochimie 2018, 146, 20–27.
- Cheng, Y.; Cheng, M.; Hao, J.; Jia, G.; Li, C. Fluorescence Spectroscopic Insight into the Supramolecular Interactions in DNA-Based Enantioselective Sulfoxidation. ChemBioChem 2018, 19, 2233–2240.
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618.
- Otocka, S.; Kwiatkowska, M.; Madalińska, L.; Kiełbasiński, P. Chiral Organosulfur Ligands/Catalysts with a Stereogenic Sulfur Atom: Applications in Asymmetric Synthesis. Chem. Rev. 2017, 117, 4147–4181.
- Mahy, J.P.; Maréchal, J.D.; Ricoux, R. From “hemoabzymes” to “hemozymes”: Towards new biocatalysts for selective oxidations. Chem. Commun. 2015, 51, 2476–2494.
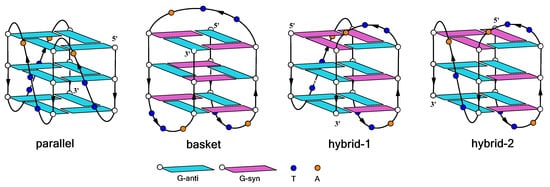
- Ambrus, A.; Chen, D.; Dai, J.; Bialis, T.; Jones, R.A.; Yang, D. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Res. 2006, 34, 2723–2735.
