The calyxes and fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino (P. alkekengi), a medicinal and edible plant, are frequently used as heat-clearing and detoxifying agents in thousands of Chinese medicine prescriptions. For thousands of years in China, they have been widely used in clinical practice to treat throat disease, hepatitis, and bacillary dysentery.
- the calyxes and fruits of P. alkekengi
- structural analysis
- quality control
- pharmacology
- pharmacokinetics
1. Introduction
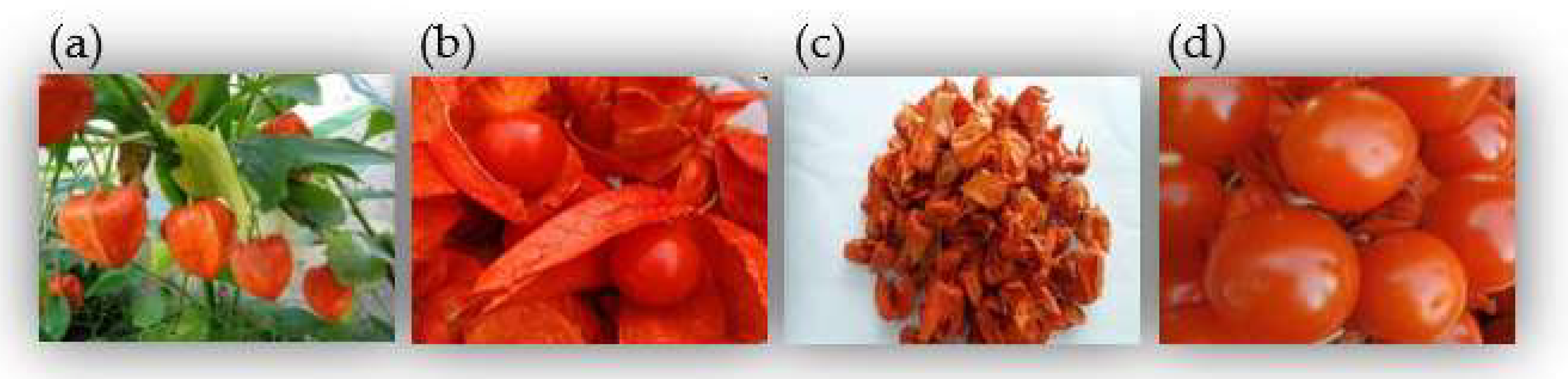 Figure 1. Images of P. alkekeng. (a) The whole plant; (b) Calyxes and fruits; (c) Calyxes; (d) Fruits.
Figure 1. Images of P. alkekeng. (a) The whole plant; (b) Calyxes and fruits; (c) Calyxes; (d) Fruits.2. Pharmacology
| Pharmacological Activity | Animal/Cell Models | Constituent/Extract | Detail | Dosage | Reference |
|---|---|---|---|---|---|
| Anti-inflammatory activity | LPS-induced 264.7 cells | Physalins A, O, L, G Isophysalin A | Induced NO production | 20 μM | [8] |
| IFN-γ-stimulated macrophages LPS-stimulated macrophages |
Physalins B, F, G | Reduced NO production; inhibited TNF-α, IL-6, IL-12 | 2 μg/mL | [9] | |
| C57BL/6 mice | Physalins B, F | Suppressed the increase in TNF-α; increased vascular permeability; prevented neutrophil influx | 20 mg/kg | [10] | |
| LPS-induced 264.7 cells | Physalin B | Decreased the levels of TNF-α, IL-6, IL-1β | 0.25, 0.5, 1.0 μM | [11] | |
| LPS/IFN-γ-induced macrophages IL-4/IL-13-induced macrophages LPS-induced C57BL/6 mice |
Physalin D | In vitro: activated signal transducer and activator of STAT6 pathway; suppressed STAT1 activation; blocked STAT1 nuclear translocation In vivo: reduced inducible iNOS cell number; increased CD206+ cell number |
5 μM | [12] | |
| LPS-stimulated RAW 264.7 cells | Physalin E | Inhibited the generation of TNF-α, IL-6, NF-κB p65; reduced the degradation of I-kappa B protein | 12.5, 25, 50 μM | [13] | |
| TPA-induced acute ear edema in mice Oxazolone-induced chronic dermatitis in mice |
Physalin E | Reduced ear edema response and myeloperoxidase activity; suppressed increase in ear thickness and levels of TNF-α and IFN-γ | 0.125, 0.25, 0.5 mg/ear | [14] | |
| DBA/1 mice | Physalin F | Decreased paw edema and joint inflammation | 60 mg/kg | [15] | |
| LPS-induced macrophages | Physalin X Aromaphysalin B |
Inhibited NO production | IC50 = 68.50, 29.69 μM, respectively | [16] | |
| LPS-induced macrophages | Physalins B, F, H, V, D1, VII, I Isophysalin B |
Inhibited NO production | IC50 = 0.32–4.03, 12.83–34.19 μM, respectively. | [17] | |
| LPS-induced macrophages | Physalins A, B, F Ombuine Luteolin |
Inhibited NO production | IC50 = 2.57 ± 1.18, 0.84 ± 0.64, 0.33 ± 0.17, 2.23 ± 0.19, 7.39 ± 2.18 µM, respectively. | [18] | |
| LPS/IFN-γ-stimulated macrophages ICR mice |
Luteolin | In vitro: suppressed the production of IL-6, IL-12, and TNF-α In vivo: inhibited paw edema |
20 μM 20 mg/kg |
[19] | |
| KF-8 cells | Apigenin Lutelin |
Inhibited NF-κB activation and the expression of CCL2/MCP-1 and CXCL1/KC | 20 μM | [20] | |
| LPS-induced macrophages | Kaempferol Quercetin |
Inhibited STAT-1 and NF-κB activation, iNOS protein and mRNA expression, and NO production | 100 μM | [21][22] | |
| LPS-stimulated THP-1 cells ICR mice |
70% ethanol extract | In vitro: reduced the production of NO, PGE2, TNF-α, IL-1, iNOS, and COX-2 In vivo: reduced ear edema; induced granulomatous tissue formation |
500 μg/mL | [23] | |
| Wistar rats | Methanol extract | Reduced the paw volume | 500 mg/kg | [24] | |
| LPS-induced macrophages | Physanosides B | Inhibited NO production | IC50 = 9.93 μM | [25] | |
| LPS-induced macrophages | (6S,9R)-roseoside | Inhibited NO production | IC50 = 7.31 μM | [26] | |
| Anti-tumor activity | HepG2 cells | Physalin A | Activated the Nrf2–ARE pathway and its target genes | 20 μM | [26] |
| Non-small cell lung cancer BALB /c mice |
Physalin A | In vitro: suppressed both constitutive and induced STAT3 activity In vivo: suppressed tumor xenograft growth |
5,10, 15 μM 40, 80 mg/kg |
[27] | |
| Human melanoma A375-S2 cells | Physalin A | Activated transmembrane death receptor; Induced poptosis via apoptotic (intrinsic and extrinsic) pathway; up-regulated p53-NOXA-mediated ROS generation |
15 μM | [28] | |
| Human HT1080 fibrosarcoma cells | Physalin A | Upregulated CASP3, CASP8 expression | IC50 = 10.7 ± 0.91 μM | [29] | |
| Human melanoma A375-S2 cells | Physalin A | Repressed the production of RNS and ROS; triggered the expression of iNOS and NO | 15 μM | [30] | |
| Non-small cell lung cancer | Physalin A | Induced G2/M cell cycle arrest; increased the amount of intracellular ROS | IC50 = 28.4 μM | [31] | |
| Prostate cancer cells (CWR22Rv1, C42B) | Physalins A, B | Inhibited the growth of two cells; activated the JNK and ERK pathway | IC50 = 14.2, 9.6 μM, respectively | [32] | |
| Non-small cell lung cancer | Physalin B | Exhibited anti-proliferative and apoptotic activity; downregulated the CDK1/CCNB1 complex; upregulated p21 | 5, 10, 20 μmol/L | [33] | |
| Human melanoma A375 cells | Physalin B | Activated the expression of the NOXA, BCL2 associated X (Bax), and CASP3 | 3 μg/mL | [34] | |
| Human HCT116 colon cancer cells | Physalin B | Activated the ERK, JNK, and p38 MAPK pathways; increased ROS generation | IC50 = 1.35 μmol/L | [35] | |
| Human DLD-1 colon cancer cells | Physalin B | Inhibited TNFα-induced NF-κB activation; induced the proapoptotic protein NOXA generation | 5 μM | [36] | |
| Breast cancer cells (MCF-7, MDA-MB-231, T-47D) | Physalin B | Induced cell cycle arrest at G2/M phase; promoted the cleavage of PARP, CASP3, CASP7, and CASP9; inactivated Akt and P13K phosphorylation | 2.5, 5, 10 μM | [37] | |
| TNF-α-stimulated HeLa cells | Physalins B, C, F | Inhibited the phosphorylation and degradation of IκBα and NF-κB activation | IC50 = 6.07, 6.54, 2.53 μM, respectively | [38] | |
| Tumor cells (A549, K562) | (17S,20R,22R)-5β,6β-epoxy-18,20-dihydroxy-1-ox- owitha-2,24-dienolide withaphysalin B |
Suppressed the PI3K/Akt/mTOR signaling pathway | IC50 = 1.9–4.3 μM | [39] | |
| Tumor cells (B-16, HCT-8, PC3, MDA-MB-435, MDA-MB-231, MCF-7, K562, CEM, HL-60) Swiss mice |
Physalins B, D | In vitro: displayed activity against several cancer cell lines In vivo: inhibited the proliferation of cells; reduced Ki67 staining |
0.58–15.18, 0.28–2.43 μg/mL, respectively 10, 25 mg/kg |
[40] | |
| Human cancer cells (C4-2B, 22Rv1, 786-O, A-498, ACHN, A375-S2) | Physalins B, F | Showed anti-proliferative activities | IC50 = 0.24–3.17 μM | [17] | |
| Human T cell leukemia Jurkat cells | Physalins B, F | Inhibited PMA-induced NF-κB and TNF-α-induced NF-κB activation | 8, 16 µM, respectively | [41] | |
| HEK293T cells BALB/c-nu/nu mice |
Physalin F | In vitro: decreased TOPFlash reporter activity; promoted the proteasomal degradation of β-catenin In vivo: downregulated β-catenin |
4 μM 10, 20 mg/kg |
[42] | |
| T-47D cells | Physalin F | Activated the CASP3 and c-myc pathways | IC50 = 3.60 μg/mL | [43] | |
| Human renal, carcinoma cells (A498, ACHN, UO-31) | Physalin F | Induced cell apoptosis through the ROS-mediated mitochondrial pathway; suppressed NF-κB activation | 1, 3, 10 μg/mL | [44] | |
| PC-3 cancer cell lines | 7β-ethoxyl-isophysalin C | Showed apparent moderate activities | IC50 = 8.26 µM | [45] | |
| Human osteosarcoma cells | Physakengose G | Inhibited the epidermal growth factor receptor/mTOR (EGFR/mTOR) pathway; blocked autophagic flux through lysosome dysfunction | 5, 10, 20 μM | [46] | |
| Immunosuppressive activity | Trypanosoma cruzi (T. cruzi)-infected insects | Physalin B | Decreased number of T. cruzi Dm28c and T. cruzi transmission; inhibited the development of parasites | 1 mg/mL 20 ng 57 ng/cm2 |
[47] |
| H14 Trypanosoma rangeli-infected Rhodnius prolixus larvae | Physalin B | Reduced the production of hemocyte microaggregation and NO | 0.1, 1 μg/mL | [48] | |
| T. cruzi trypomastigotes BALB/c mice macrophages |
Physalin B Physalin F |
Displayed strongest effects against epimastigote forms of T. cruzi | IC50 = 5.3 ± 1.9, 5.8 ± 1.5 μM, respectively IC50 = 0.68 ± 0.01, 0.84 ± 0.04 μM, respectively |
[49] | |
| Con A-induced spleen cells CBA mice |
Physalins B, F, G | In vitro: inhibited MLR and IL-2 production In vivo: prevented the rejection of allogeneic heterotopic heart transplant |
2 μg/mL 1 mg/mouse/day |
[50] | |
| Human T-cell lymphotropic virus type 1 (HTLV-1)-infected subjects | Physalin F | Inhibited spontaneous proliferation; reduced the levels of IL-2, IL-6, IL-10, TNF-α, and IFN-γ | 10 μM | [51] | |
| T cells BALB/c mice |
Physalin H | In vitro: suppressed proliferation and MLR In vivo: inhibited delayed-type hypersensitivity reactions and T-cell response |
IC50 = 0.69, 0.39 μg/mL, respectively IC50 = 2.75 or 3.61 μg/mL |
[52] | |
| ICR mice | Polysaccharides | Enhanced specific antibody titers immunoglobulin G (IgG), IgG1, and IgG2b, as well as the concentration of IL-2 and IL-4 | 40 µg/mice | [53] | |
| Anti-microbial activity | Gram-positive bacteria: Staphylococcus epidermidis (S. epidermidis), Enterococcus faecalis (E. faecalis), Staphylococcus aureus (S. aureus), Bacillus subtilis (B. subtilis), Bacillus cereus (B. cereus) | Methanol extract Dichloromethane extract Physalin D |
Displayed moderate antibacterial activity | MIC = 32–128 µg/mL | [54] |
| Escherichia coli (E. coli), B. subtilis | Physalins B, J, P | Showed high antibacterial activity | MIC = 12.5–23.7, 23.23–24.34, 22.8–27.98 µg/mL, respectively | [55] | |
| Mycobacterium tuberculosis H37Rv | Trichlormethane extract Physalins B, D |
Showed antibacterial activity | MIC = 32, >128, 32 µg/mL, respectively | [56] | |
| Lactobacillus delbrueckii (L. delbrueckii), E. coli |
70% ethanol extract | Promoted the growth of L. delbrueckii; inhibited the growth of E. coli | 0.78–1.56 mg/mL | [57] | |
| Gram-positive bacteria: S. aureus, S. epidermidis, Staphylococcus saprophyticus (S. saprophyticus), Enterococcus faecium (E. faecium) Gram-negative bacteria: Pseudomonas aeruginosa (P. aeruginosa), Streptococcus pneumoniae (S. pneumoniae), E. coli |
70% ethanol extract | Showed antibacterial activity | MIC = 0.825–1.65 mg/mL | [23] | |
| S. aureus, B. subtilis, P. aeruginosa, E. coli | Physakengoses B, E, F, G, H, K, L, M, N, O | Showed potent inhibitory effects | MIC = 2.16–14.9 μg/mL | [58][59] | |
| Anti-leishmanial | Leishmania-infected macrophages Leishmania amazonensis -infected BALB/c mice |
Physalins B, F | In vitro: reduced the percentage of macrophages In vivo: reduced the lesion size, the parasite load, and histopathological alterations |
IC50 = 0.21 and 0.18 μM, respectively | [60] |
| Others | Kunming mice | Water extract | Decreased the expression of white blood cells and eosinophils, IL-5, IFN-γ, Th1, and Th2 | 0.25, 5, 1 g/mL | [61] |
| 3T3-L1 pre-adipocyte cells HepG2 cells Male Sprague–Dawley (SD) rats |
Ethyl acetate extract | In vitro: relieved oxidative stress; inhibited α-glucosidase activity. In vivo: decreased FBG, TC, and TG |
300 mg/kg | [62] | |
| Alloxan-induced mice | Polysaccharides | Decreased FBG and GSP; increased FINS; upregulated the PI3K, Akt, and GLUT4 mRNA | 200, 400, 800 mg/kg | [63] | |
| High-fat diet-fed and streptozotocin-induced diabetic SD rats | Ethyl acetate extract | Reduced the FBG, TC, TG, and GSP; increased FINS | 300, 600 mg/kg | [64] | |
| Wistar rats Albino mice |
Aqueous methanolic extract | Reduced the intensity of gastric mucosal damage; inhibited pain sensation | 500 μg/mL 500 mg/kg |
[24] | |
| LPS-induced acute lung injury in BALB/c mice | 70% ethanol extract | Reduced the release of TNF-α and the accumulation of oxidation products; decreased the levels of NF-κB, phosphorylated-p38, ERK, JNK, p53, CASP3, and COX-2 | 500 mg/kg | [65] | |
| 4% dextran sulfate sodium--induced colitis in BALB/c mice | Physalin B | Reduced MPO activity; suppressed the activation of NF-κB, STAT3, arrestin beta 1 (ARRB1), and NLR family pyrin domain containing 3 (NLRP3) | 10, 20 mg/kg | [11] | |
| N2a/APPsw cells | Physalin B | Downregulated β-amyloid (Aβ) secretion and the expression of beta-secretase 1 (BACE1) | 3 μmol/L | [66] | |
| DPPH TBA |
Physalin D | Exhibited antioxidant activity | IC50 ≥ 10 ± 2.1 µg/mL | [54] | |
| Plasmodium berghei-infected mice | Physalins B, D, F, G | Caused parasitemia reduction and delay | 50, 100 mg/kg | [67] | |
| High glucose-induced primary mouse hepatocytes Oleic acid-induced HepG2 cells Kunming mice |
75% ethanol extract Luteolin-7-O-β-d-glucopyranoside |
In vitro: decreased the levels of TG in HepG2 cells In vivo: decreased the levels of TC and TG |
50, 100 μg/mL, respectively 1 or 2 g/kg, 0.54 g/kg, respectively |
[68] | |
| SD mice | Luteolin | Increased NO; activated PI3K/Akt/NO signaling pathway; enhanced the activity of endothelial NOS | 7.5 µg/mL | [69] | |
| SD rats | Luteolin | Conferred a cardioprotective effect; ameliorated Ca2+ overload | 7.5, 15, 30 μmol/L | [70] |
2.1. Anti-Inflammatory Activity
2.2. Anti-Tumor Activity
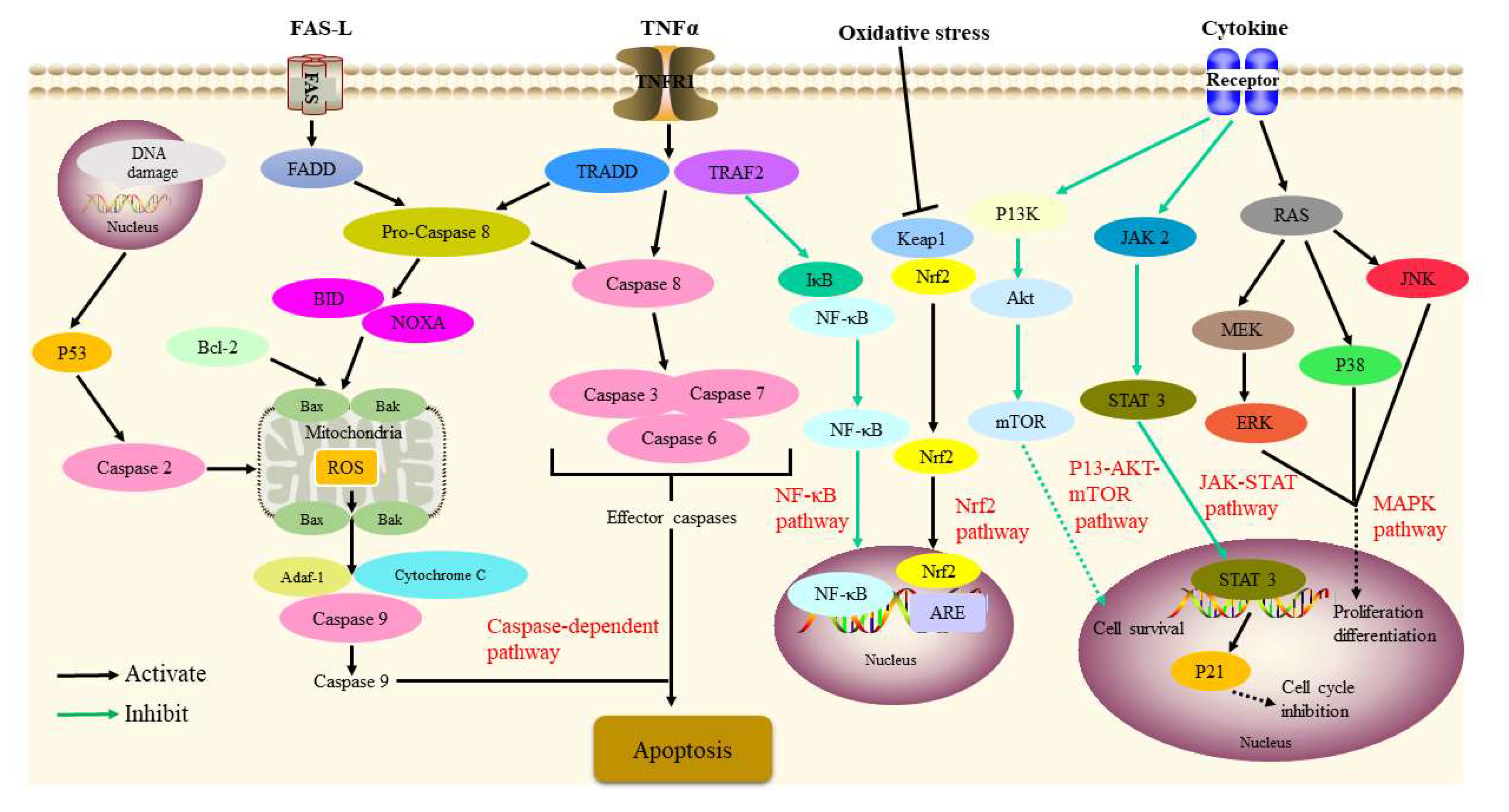 Figure 2. Signaling pathways involved in the antitumor activity of P. alkekengi and its constituents.
Figure 2. Signaling pathways involved in the antitumor activity of P. alkekengi and its constituents.2.3. Immunosuppressive Activity
2.4. Antibacterial Activity
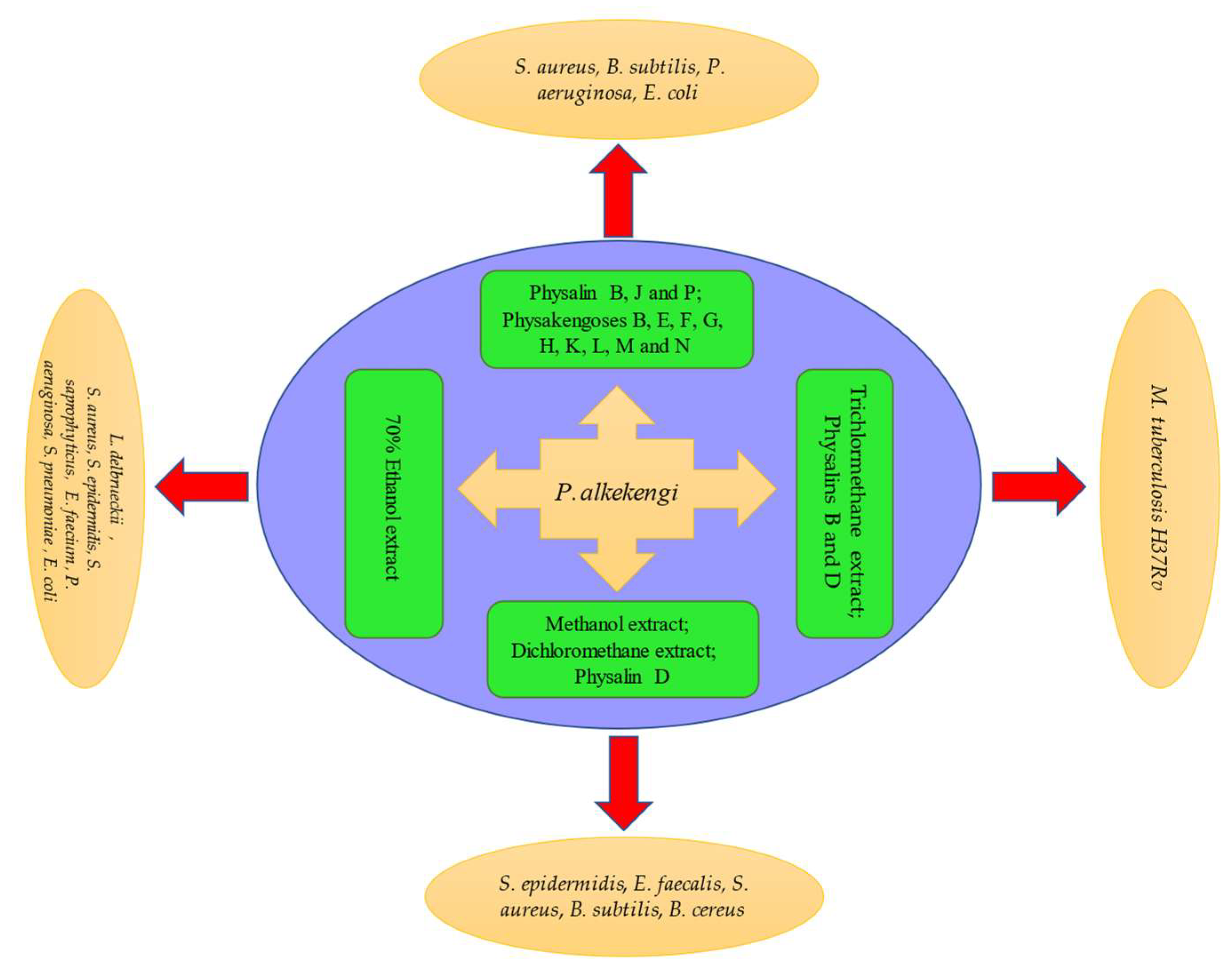 Figure 3. Schematic representation of antibacterial activity of P. alkekengi and its constituents.
Figure 3. Schematic representation of antibacterial activity of P. alkekengi and its constituents.2.5. Antileishmanial Activity
2.6. Others
3. Summary
In summary, P. alkekengi is an excellent, abundant, inexpensive, and edible drug. The synthesis of the main active components of P. alkekengi must be further analyzed using additional biological and chemical techniques to further expand their potential applications. In addition, the quantitative analysis of the chemical constituents of P. alkekengi should be employed for the purpose of standardization and quality control of extracts. Lastly, additional in vivo animal research and clinical trials are needed to determine whether various applications of P. alkekengi are effective and safe in a larger population.
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part I; People’s Medical Publishing House: Beijing, China, 2020; p. 360. (In Chinese)
- Zheng, W.J.; Fu, L.G. Flora of China; Editorial Committee of Flora of China, Chinese Academy of Sciences, Science Press: Beijing, China, 1978; p. 54. (In Chinese)
- Shu, Z.P.; Xu, B.Q.; Xing, N.; Li, X.L.; Wang, Q.H.; Yang, B.Y.; Kuang, H.X. Chemical constituents of Physalis Calyx seu Fructus. Zhong Guo Shi Yan Fang Ji Xue Za Zhi 2014, 20, 99–102.
- Gao, P.Y.; Jin, M.; Du, C.L.; Liu, X.G. Research progress of Physalis alkekengi var. franchetii. Shenyang Yao Ke Da Xue Xue Bao 2014, 31, 732–737.
- Wen, X.; Erşan, S.; Li, M.; Wang, K.; Steingass, C.B.; Schweiggert, R.M.; Ni, Y.; Carle, R. Physicochemical characteristics and phytochemical profiles of yellow and red Physalis (Physalis alkekengi L. and P.pubescens L.) fruits cultivated in China. Food Res. Int. 2019, 120, 389–398.
- Li, A.L.; Chen, B.J.; Li, G.H.; Zhou, M.X.; Li, Y.R.; Ren, D.M.; Lou, H.X.; Wang, X.N.; Shen, T. Physalis alkekengi L. var. franchetii (Mast.) Makino: An ethnomedical, phytochemical and pharmacological review. J. Ethnopharmacol. 2018, 210, 260–274.
- Yang, L.J.; Wang, D.D.; Wu, H.J.; Chen, D.Z. Study on the action targets for anti-inflammatory bioactive components of Physalis alkekengi L. var. franchetii (Mast.) Makino based on network pharmacology. J. Tianjin Univ. Tradit. Chin. Med. 2018, 37, 399–403.
- Ji, L.; Yuan, Y.; Luo, L.; Chen, Z.; Ma, X.; Ma, Z.; Cheng, L. Physalins with anti-inflammatory activity are present in Physalis alkekengi var. franchetii and can function as michael reaction acceptors. Steroids 2012, 77, 441–447.
- Soares, M.B.; Bellintani, M.C.; Ribeiro, I.M.; Tomassini, T.C.; dos Santos, R.R. Inhibition of macrophage activation and lipopolysaccaride-induced death by seco-steroids purified from Physalis angulata L. Eur. J. Pharmacol. 2003, 459, 107–112.
- Vieira, A.T.; Pinho, V.; Lepsch, L.B.; Scavone, C.; Ribeiro, I.M.; Tomassini, T.; Ribeiro-dos-Santos, R.; Soares, M.B.; Teixeira, M.M.; Souza, D.G. Mechanisms of the anti-inflammatory effects of the natural secosteroids physalins in a model of intestinal ischaemia and reperfusion injury. Br. J. Pharmacol. 2005, 146, 244–251.
- Zhang, Q.; Xu, N.; Hu, X.; Zheng, Y. Anti-colitic effects of physalin B on dextran sodium sulfate-induced BALB/c mice by suppressing multiple inflammatory signaling pathways. J. Ethnopharmacol. 2020, 259, 112956.
- Ding, N.; Wang, Y.; Dou, C.; Liu, F.; Guan, G.; Wei, K.; Yang, J.; Yang, M.; Tan, J.; Zeng, W.; et al. Physalin D regulates macrophage M1/M2 polarization via the STAT1/6 pathway. J. Cell. Physiol. 2019, 234, 8788–8796.
- Yang, Y.J.; Yi, L.; Wang, Q.; Xie, B.B.; Dong, Y.; Sha, C.W. Anti-inflammatory effects of physalin E from Physalis angulata on lipopolysaccharide-stimulated RAW 264.7 cells through inhibition of NF-κB pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 74–79.
- Pinto, N.B.; Morais, T.C.; Carvalho, K.M.; Silva, C.R.; Andrade, G.M.; Brito, G.A.; Veras, M.L.; Pessoa, O.D.; Rao, V.S.; Santos, F.A. Topical anti-inflammatory potential of physalin E from Physalis angulata on experimental dermatitis in mice. Phytomedicine 2010, 17, 740–743.
- Brustolim, D.; Vasconcelos, J.F.; Freitas, L.A.; Teixeira, M.M.; Farias, M.T.; Ribeiro, Y.M.; Tomassini, T.C.; Oliveira, G.G.; Pontes-de-Carvalho, L.C.; Ribeiro-dos-Santos, R.; et al. Activity of physalin F in a collagen-induced arthritis model. J. Nat. Prod. 2010, 73, 1323–1326.
- Sun, C.P.; Oppong, M.B.; Zhao, F.; Chen, L.X.; Qiu, F. Unprecedented 22,26-seco physalins from Physalis angulata and their anti-inflammatory potential. Org. Biomol. Chem. 2017, 15, 8700–8704.
- Sun, C.P.; Qiu, C.Y.; Zhao, F.; Kang, N.; Chen, L.X.; Qiu, F. Physalins V-IX, 16,24-cyclo-13,14-seco withanolides from Physalis angulata and their antiproliferative and anti-inflammatory activities. Sci. Rep. 2017, 7, 4057.
- Qiu, L.; Zhao, F.; Jiang, Z.H.; Chen, L.X.; Zhao, Q.; Liu, H.X.; Yao, X.S.; Qiu, F. Steroids and flavonoids from Physalis alkekengi var. franchetii and their inhibitory effects on nitric oxide production. J. Nat. Prod. 2008, 71, 642–646.
- Ziyan, L.; Yongmei, Z.; Nan, Z.; Ning, T.; Baolin, L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Medica 2007, 73, 221–226.
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-inflammatory activity of structurally related flavonoids, apigenin, luteolin and fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159.
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 045673.
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245.
- Shu, Z.; Xing, N.; Wang, Q.; Li, X.; Xu, B.; Li, Z.; Kuang, H.X. Antibacterial and anti-inflammatory activities of Physalis alkekengi var. franchetii and its main constituents. Evid.-Based Complement. Altern. Med. 2016, 2016, 4359394.
- Wang, Y.; Wang, S.L.; Zhang, J.Y.; Song, X.N.; Zhang, Z.Y.; Li, J.F.; Li, S. Anti-ulcer and anti-Helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent. J. Ethnopharmacol. 2018, 211, 197–206.
- Qiu, L.; Zhao, F.; Liu, H.; Chen, L.; Jiang, Z.; Liu, H.; Wang, N.; Yao, X.; Qiu, F. Two New Megastigmane Glycosides, Physanosides A and B, from Physalisalkekengi L. var. franchetii, and Their Effect on NO Release in Macrophages. Chem. Biodivers. 2008, 5, 758–763.
- Shin, J.M.; Lee, K.M.; Lee, H.J.; Yun, J.H.; Nho, C.W. Physalin A regulates the Nrf2 pathway through ERK and p38 for induction of detoxifying enzymes. BMC Complement. Altern. Med. 2019, 19, 101.
- Zhu, F.; Dai, C.; Fu, Y.; Loo, J.F.; Xia, D.; Gao, S.P.; Ma, Z.; Chen, Z. Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling. Oncotarget 2016, 7, 9462–9476.
- He, H.; Zang, L.H.; Feng, Y.S.; Chen, L.X.; Kang, N.; Tashiro, S.; Onodera, S.; Qiu, F.; Ikejima, T. Physalin A induces apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays a protective role against apoptosis through p38-NF-kappaB survival pathway in A375-S2 cells. J. Ethnopharmacol. 2013, 148, 544–555.
- He, H.; Zang, L.H.; Feng, Y.S.; Wang, J.; Liu, W.W.; Chen, L.X.; Kang, N.; Tashiro, S.; Onodera, S.; Qiu, F.; et al. Physalin A induces apoptotic cell death and protective autophagy in HT1080 human fibrosarcoma cells. J. Nat. Prod. 2013, 76, 880–888.
- He, H.; Feng, Y.S.; Zang, L.H.; Liu, W.W.; Ding, L.Q.; Chen, L.X.; Kang, N.; Hayashi, T.; Tashiro, S.; Onodera, S.; et al. Nitric oxide induces apoptosis and autophagy; autophagy down-regulates NO synthesis in physalin A-treated A375-S2 human melanoma cells. Food Chem. Toxicol. 2014, 71, 128–135.
- Kang, N.; Jian, J.F.; Cao, S.J.; Zhang, Q.; Mao, Y.W.; Huang, Y.Y.; Peng, Y.F.; Qiu, F.; Gao, X.M. Physalin A induces G2/M phase cell cycle arrest in human non-small cell lung cancer cells: Involvement of the p38 MAPK/ROS pathway. Mol. Cell. Biochem. 2016, 415, 145–155.
- Han, H.; Qiu, L.; Wang, X.; Qiu, F.; Wong, Y.; Yao, X. Physalins A and B inhibit androgen-independent prostate cancer cell growth through activation of cell apoptosis and downregulation of androgen receptor expression. Biol. Pharm. Bull. 2011, 34, 1584–1588.
- Cao, C.; Zhu, L.; Chen, Y.; Wang, C.H.; ShenTu, J.Z.; Zheng, Y.L. Physalin B induces G2/M cell cycle arrest and apoptosis in A549 human non-small-cell lung cancer cells by altering mitochondrial function. Anti-Cancer Drugs 2019, 30, 128–137.
- Hsu, C.C.; Wu, Y.C.; Farh, L.; Du, Y.C.; Tseng, W.K.; Wu, C.C.; Chang, F.R. Physalin B from Physalis angulata triggers the NOXA-related apoptosis pathway of human melanoma A375 cells. Food Chem. Toxicol. 2012, 50, 619–624.
- Ma, Y.M.; Han, W.; Li, J.; Hu, L.H.; Zhou, Y.B. Physalin B not only inhibits the ubiquitin-proteasome pathway but also induces incomplete autophagic response in human colon cancer cells in vitro. Acta Pharmacol. Sin. 2015, 36, 517–527.
- Vandenberghe, I.; Créancier, L.; Vispé, S.; Annereau, J.P.; Barret, J.M.; Pouny, I.; Samson, A.; Aussagues, Y.; Massiot, G.; Ausseil, F.; et al. Physalin B, a novel inhibitor of the ubiquitin-proteasome pathway, triggers NOXA-associated apoptosis. Biochem. Pharmacol. 2008, 76, 453–462.
- Wang, A.; Wang, S.; Zhou, F.; Li, P.; Wang, Y.; Gan, L.; Lin, L. Physalin B induces cell cycle arrest and triggers apoptosis in breast cancer cells through modulating p53-dependent apoptotic pathway. Biomed. Pharmacother. 2018, 101, 334–341.
- Ozawa, M.; Morita, M.; Hirai, G.; Tamura, S.; Kawai, M.; Tsuchiya, A.; Oonuma, K.; Maruoka, K.; Sodeoka, M. Contribution of Cage-Shaped Structure of Physalins to Their Mode of Action in Inhibition of NF-κB Activation. ACS Med. Chem. Lett. 2013, 4, 730–735.
- Sun, Y.; Guo, T.; Zhang, F.B.; Wang, Y.N.; Liu, Z.; Guo, S.; Li, L. Isolation and characterization of cytotoxic withanolides from the calyx of Physalis alkekengi L. var franchetii. Bioorg. Chem. 2020, 96, 103614.
- Magalhães, H.I.; Veras, M.L.; Torres, M.R.; Alves, A.P.; Pessoa, O.D.; Silveira, E.R.; Costa-Lotufo LV de Moraes, M.O.; Pessoa, C. In-vitro and in-vivo antitumour activity of physalins B and D from Physalis angulata. J. Pharm. Pharmacol. 2006, 58, 235–241.
- Jacobo-Herrera, N.J.; Bremner, P.; Marquez, N.; Gupta, M.P.; Gibbons, S.; Muñoz, E.; Heinrich, M. Physalins from Witheringia solanacea as modulators of the NF-kappaB cascade. J. Nat. Prod. 2006, 69, 328–331.
- Chen, C.; Zhu, D.; Zhang, H.; Han, C.; Xue, G.; Zhu, T.; Luo, J.; Kong, L. YAP-dependent ubiquitination and degradation of beta-catenin mediates inhibition of Wnt signalling induced by physalin F in colorectal cancer. Cell Death Dis. 2018, 9, 591.
- Ooi, K.L.; Muhammad, T.S.; Sulaiman, S.F. Physalin F from Physalis minima L. triggers apoptosis-based cytotoxic mechanism in T-47D cells through the activation caspase-3- and c-myc-dependent pathways. J. Ethnopharmacol. 2013, 150, 382–388.
- Wu, S.Y.; Leu, Y.L.; Chang, Y.L.; Wu, T.S.; Kuo, P.C.; Liao, Y.R.; Teng, C.M.; Pan, S.L. Physalin F induces cell apoptosis in human renal carcinoma cells by targeting NF-kappaB and generating reactive oxygen species. PLoS ONE 2012, 7, e40727.
- Sun, J.L.; Jiang, Y.J.; Cheng, L. Two new physalin derivatives from Physalis alkekengi L. var. franchetii (Mast.) Makino. Nat. Prod. Res. 2021, 35, 203–206.
- Lin, H.; Zhang, C.; Zhang, H.; Xia, Y.Z.; Zhang, C.Y.; Luo, J.; Yang, L.; Kong, L.Y. Physakengose G induces apoptosis via EGFR/mTOR signaling and inhibits autophagic flux in human osteosarcoma cells. Phytomedicine 2018, 42, 190–198.
- Castro, D.P.; Moraes, C.S.; Gonzalez, M.S.; Ribeiro, I.M.; Tomassini, T.C.; Azambuja, P.; Garcia, E.S. Physalin B inhibits Trypanosoma cruzi infection in the gut of Rhodnius prolixus by affecting the immune system and microbiota. J. Insect Physiol. 2012, 58, 1620–1625.
- Garcia, E.S.; Castro, D.P.; Ribeiro, I.M.; Tomassini, T.C.; Azambuja, P. Trypanosoma rangeli: Effects of physalin B on the immune reactions of the infected larvae of Rhodnius prolixus. Exp. Parasitol. 2006, 112, 37–43.
- Meira, C.S.; Guimarães, E.T.; Bastos, T.M.; Moreira, D.R.; Tomassini, T.C.; Ribeiro, I.M.; Dos Santos, R.R.; Soares, M.B. Physalins B and F, seco-steroids isolated from Physalis angulata L., strongly inhibit proliferation, ultrastructure and infectivity of Trypanosoma cruzi. Parasitology 2013, 140, 1811–1821.
- Soares, M.B.; Brustolim, D.; Santos, L.A.; Bellintani, M.C.; Paiva, F.P.; Ribeiro, Y.M.; Tomassini, T.C.; Dos Santos, R.R. Physalins B, F and G, seco-steroids purified from Physalis angulata L., inhibit lymphocyte function and allogeneic transplant rejection. Int. Immunopharmacol. 2006, 6, 408–414.
- Pinto, L.A.; Meira, C.S.; Villarreal, C.F.; Vannier-Santos, M.A.; de Souza, C.V.; Ribeiro, I.M.; Tomassini, T.C.; Galvão-Castro, B.; Soares, M.B.; Grassi, M.F. Physalin F, a seco-steroid from Physalis angulata L., has immunosuppressive activity in peripheral blood mononuclear cells from patients with HTLV1-associated myelopathy. Biomed. Pharmacother. 2016, 79, 129–134.
- Yu, Y.; Sun, L.; Ma, L.; Li, J.; Hu, L.; Liu, J. Investigation of the immunosuppressive activity of physalin H on T lymphocytes. Int. Immunopharmacol. 2010, 10, 290–297.
- Yang, H.; Han, S.; Zhao, D.; Wang, G. Adjuvant effect of polysaccharide from fruits of Physalis alkekengi L. in DNA vaccine against systemic candidiasis. Carbohydr. Polym. 2014, 109, 77–84.
- Helvacı, S.; Kökdil, G.; Kawai, M.; Duran, N.; Duran, G.; Güvenç, A. Antimicrobial activity of the extracts and physalin D from Physalis alkekengi and evaluation of antioxidant potential of physalin D. Pharm. Biol. 2010, 48, 142–150.
- Yang, Y.K.; Xie, S.D.; Xu, W.X.; Nian, Y.; Liu, X.L.; Peng, X.R.; Ding, Z.T.; Qiu, M.H. Six new physalins from Physalis alkekengi var. franchetii and their cytotoxicity and antibacterial activity. Fitoterapia 2016, 112, 144–152.
- Januário, A.H.; Filho, E.R.; Pietro, R.C.; Kashima, S.; Sato, D.N.; França, S.C. Antimycobacterial physalins from Physalis angulata L. (Solanaceae). Phytother. Res. 2002, 16, 445–448.
- Li, X.; Zhang, C.; Wu, D.; Tang, L.; Cao, X.; Xin, Y. In vitro effects on intestinal bacterium of physalins from Physalis alkekengi var. Francheti. Fitoterapia 2012, 83, 1460–1465.
- Zhang, C.Y.; Luo, J.G.; Liu, R.H.; Lin, R.; Yang, M.H.; Kong, L.Y. 1H NMR spectroscopy-guided isolation of new sucrose esters from Physalis alkekengi var. franchetii and their antibacterial activity. Fitoterapia 2016, 114, 138–143.
- Zhang, C.Y.; Luo, J.G.; Liu, R.H.; Lin, R.; Yang, M.H.; Kong, L.Y. Physakengoses K-Q, seven new sucrose esters from Physalis alkekengi var. franchetii. Carbohydr. Res. 2017, 449, 120–124.
- Guimarães, E.T.; Lima, M.S.; Santos, L.A.; Ribeiro, I.M.; Tomassini, T.B.; dos Santos, R.R.; dos Santos, W.L.; Soares, M.B. Activity of physalins purified from Physalis angulata in in vitro and in vivo models of cutaneous leishmaniasis. J. Antimicrob. Chemother. 2009, 64, 84–87.
- Bao, C.L. Curative Effect of Chinese Physalis Alkekeng on Mice Allergic Asthuma; Yanbian University: Yanji, China, 2008.
- Hu, X.F.; Zhang, Q.; Zhang, P.P.; Sun, L.J.; Liang, J.C.; Morris-Natschke, S.L.; Chen, Y.; Lee, K.H. Evaluation of in vitro/in vivo anti-diabetic effects and identification of compounds from Physalis alkekengi. Fitoterapia 2018, 127, 129–137.
- Guo, Y.; Li, S.; Li, J.; Ren, Z.; Chen, F.; Wang, X. Anti-hyperglycemic activity of polysaccharides from calyx of Physalis alkekengi var. franchetii Makino on alloxan-induced mice. Int. J. Biol. Macromol. 2017, 99, 249–257.
- Zhang, Q.; Hu, X.F.; Xin, M.M.; Liu, H.B.; Sun, L.J.; Morris-Natschke, S.L.; Chen, Y.; Lee, K.H. Antidiabetic potential of the ethyl acetate extract of Physalis alkekengi and chemical constituents identified by HPLC-ESI-QTOF-MS. J. Ethnopharmacol. 2018, 225, 202–210.
- Yang, Y.; Ding, Z.; Wang, Y.; Zhong, R.; Feng, Y.; Xia, T.; Xie, Y.; Yang, B.; Sun, X.; Shu, Z. Systems pharmacology reveals the mechanism of activity of Physalis alkekengi L. var. franchetii against lipopolysaccharide-induced acute lung injury. J. Cell. Mol. Med. 2020, 24, 5039–5056.
- Zhang, W.; Bai, S.S.; Zhang, Q.; Shi, R.L.; Wang, H.C.; Liu, Y.C.; Ni, T.J.; Wu, Y.; Yao, Z.Y.; Sun, Y.; et al. Physalin B reduces Aβ secretion through down-regulation of BACE1 expression by activating FoxO1 and inhibiting STAT3 phosphorylation. Chin. J. Nat. Med. 2021, 19, 732–740.
- Sá, M.S.; de Menezes, M.N.; Krettli, A.U.; Ribeiro, I.M.; Tomassini, T.C.; dos Santos, R.R.; de Azevedo, W.F.J.; Soares, M.B. Antimalarial activity of physalins B, D, F, and G. J. Nat. Prod. 2011, 74, 2269–2272.
- Yang, Y.; Piao, X.; Zhang, M.; Wang, X.; Xu, B.; Zhu, J.; Fang, Z.; Hou, Y.; Lu, Y.; Yang, B. Bioactivity-guided fractionation of the triglyceride-lowering component and in vivo and in vitro evaluation of hypolipidemic effects of Calyx seu Fructus Physalis. Lipids Health Dis. 2012, 11, 38.
- Yang, Y.; Chen, B.; Liang, K.L.; Su, J.; Chen, S.H.; Lv, G.Y. Relaxation effect of buddleoside combined with luteolin on isolated vessels in vivo and its mechanism. China J. Chin. Mater. Med. 2017, 42, 1370–1375.
- Yan, Q.; Li, Y.; Yan, J.; Zhao, Y.; Liu, Y.; Liu, S. Effects of luteolin on regulatory proteins and enzymes for myocyte calcium circulation in hypothermic preserved rat heart. Exp. Ther. Med. 2018, 15, 1433–1441.
- Tariq, A.; Adnan, M.; Amber, R.; Pan, K.; Mussarat, S.; Shinwari, Z.K. Ethnomedicines and anti-parasitic activities of Pakistani medicinal plants against Plasmodia and Leishmania parasites. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 52.
- Guimarães, E.T.; Lima, M.S.; Santos, L.A.; Ribeiro, I.M.; Tomassini, B.C.; Santos, R.R.; Santos, L.C.; Soares, B.P. Effects of seco-steroids purified from Physalis angulata L., Solanaceae, on the viability of Leishmania sp. Rev. Bras. Farmacogn. 2010, 20, 945–949.
- Tan, X.; Jin, P.; Feng, L.; Song, J.; Sun, E.; Liu, W.; Shu, L.; Jia, X. Protective effect of luteolin on cigarette smoke extract-induced cellular toxicity and apoptosis in normal human bronchial epithelial cells via the Nrf2 pathway. Oncol. Rep. 2014, 31, 1855–1862.
