Heme oxygenase 1 (HO-1), the rate-limiting enzyme in heme degradation, is involved in the maintenance of cellular homeostasis, exerting a cytoprotective role by its antioxidative and anti-inflammatory functions. HO-1 and its end products, biliverdin (BV), carbon monoxide (CO) and free iron (Fe2+), confer cytoprotection against inflammatory and oxidative injury. Additionally, HO-1 exerts antiviral properties against a diverse range of viral infections by interfering with replication or activating the interferon (IFN) pathway. Severe cases of coronavirus disease 2019 (COVID-19), an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), are characterized by systemic hyperinflammation, which, in some cases, leads to severe or fatal symptoms as a consequence of respiratory failure, lung and heart damage, kidney failure, and nervous system complications. Here we summarize the current research on the protective role of HO-1 in inflammatory diseases and against a wide range of viral infections, positioning HO-1 as an attractive target to ameliorate clinical manifestations during COVID-19.
- heme oxygenase 1
- COVID-19
- influenza A virus
- respiratory syncytial virus
- human immunodeficiency virus
- Ebola virus
- Dengue virus
- Zika virus
- SARS-CoV-2
1. Introduction
The treatment goal in COVID-19 patients is to prevent or to decrease the strong virus induced inflammatory stimuli associated with a wide spectrum of poor prognosis clinical manifestations [1]. HO-1 is a microsomal enzyme with a primary antioxidant and anti-inflammatory role involved in heme degradation, generating CO, BV, and Fe2+ [2]. Hence, HO-1 induction is a useful approach for inflammatory diseases treatment [3][4][5][6]. Additionally, HO-1 displays antiviral properties against a wide range of viruses [7]. Hemin, a previously Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved drug for acute intermittent porphyria treatment [8][9], is a well known inducer of HO-1 that increases its plasma concentration in humans. Thus, hemin rises as a promising drug candidate against the replication of different viruses, including SARS-CoV-2.
2. Cytokine Storm and Inflammation
Inflammation involves defense mechanisms against infection or injury. It is responsible for activating both innate and adaptive immune responses [10][11]. During infections, innate cells recognize pathogen associated molecular patterns from the invading agent. In the case of inflammation triggered by tissue damage, trauma or ischemia, innate cells recognize host specific molecules that are released during cell injury or necrotic death, defined as damage associated molecular patterns, such as nucleic acids and adenosine triphosphate [10]. During the early stages of inflammation, innate immune cells and endothelial cells (EC) release a diverse set of cytokines and recruit other immune cells to the site of infection or inflammation. Proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-1β [12][13], are also released and trigger the activation of inflammatory pathways, including the mitogen activated protein kinase (MAPK), nuclear factor kappa-B (NF-κB), and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathways [11]. Some pathogenic viruses (i.e., highly virulent subtypes of influenza) and bacteria (i.e., Francisella tularensis) can induce the acute dysregulated production of inflammatory cytokines, known as “cytokine storm” or hypercytokinemia [14]. The hypercytokinemia and exacerbated secondary events, such as coagulation, eventually result in widespread necrosis, organ failure and death [10][15]. Once SARS-CoV-2 infects target cells, innate immune cells are recruited to the infection site, where they release cytokines and initiate the activation cascade of adaptive B and T cell immune responses [16]. In most cases, the immune system is able to eliminate virus infected cells and resolve the immune response. However, in some patients, this process is dysfunctional, impairing the effective clearance of infected cells, and causing severe damage to the host [17].3. The Lead Role of Interferons upon Viral Infection
During viral infections, pattern recognition receptors are stimulated to produce IFN by the innate immune cells. IFNs are crucial for the induction of an antiviral state via autocrine and paracrine signaling. There are three types of IFNs: type I, type II and type III. Type I (IFNα, IFNβ, IFNω, IFNτ, IFNε) and Type III (IFNλ1, IFNλ2/3, IFNλ4) share similar dynamics after binding to its receptor, as cross-phosphorylation between JAK1 and tyrosine kinase 2 (TYK2) occurs [18]. Subsequently, a docking site for STAT1 is exposed, STAT1 is phosphorylated, translocates to the nucleus, and induces the transcription of interferon stimulated genes (ISGs). The IFNs biological effects are wide, including immuno-regulation, antiviral, anti-angiogenic, and pro-apoptotic functions [19]. However, many pathogens have evolved to elude the action mechanisms of these powerful cytokines [20][21][22].4. Understanding the Protective Role of Heme Oxygenase 1
Heme oxygenases (HO) are metabolic enzymes that partake in the degradation of the heme group [2]. To date, three isoforms of this protein have been found: HO-1, which can be induced by external factors (such as hypoxia, oxidative stress, heat shock, reactive oxygen species (ROS), among others) [23]; HO-2, a constitutively expressed isoform; and HO-3, a non-functional isoform in humans [24]. In particular, HO-1, encoded by the HMOX1 gene, is involved in the maintenance of cellular homeostasis, exerting a cytoprotective role by its anti-inflammatory, anti-oxidative and anti-apoptotic functions, as revealed in a human case of genetic HO-1 deficiency [25]. This enzyme participates not only in normal physiological processes, but also performs a protective role in inflammatory physiopathological conditions, such as kidney disease [26], cancer [27][28], cardiovascular disease [29], asthma [30] and inflammatory bowel diseases [4][31]. HO-1 is expressed in most cell types and tissues; however, its capacity to counteract inflammation seems to be critically dependent on its specific functions in myeloid cells and in EC [32]. In myeloid cells, HO-1 acts as a key regulator of the TLR4/TLR3/IRF3 induced production of IFN-β and primary IRF3 target genes in macrophages [33] and modulates maturation and specific functions of dendritic cells [34][35]. Moreover, HO-1 over-expression in macrophages negatively regulates the expression of diverse proinflammatory molecules and increases the expression of anti-inflammatory cytokines [36][37][38]. Among HO-1 effects on EC, it is significant to mention its ability to inhibit the expression of pro-inflammatory genes related to EC activation, such as the TNF-α-induced adhesion molecules, E-selectin and VCAM-1, via a mechanism associated with the inhibition of NF-κB activation [39]. As it was mentioned before, HO-1 cleaves the heme group, which is usually bound to a myriad of proteins and it is involved in several homeostatic functions [23]. However, elevated concentrations of heme can cause cell damage because it is a pro-oxidant molecule. It can diffuse through cell membranes and deliver a redox active iron, producing ROS [40]. Excessive amounts of these molecules are toxic and induce oxidative stress that, in turn, generates DNA and protein damage, aggregation and lipid peroxidation, triggering cells permeability and driving cell lysis and death [40].5. HO-1 Mechanism of Action against Inflammatory Lung Diseases
There is extensive literature about the role of HO-1 in lung diseases. This protein is expressed in type II pneumocytes and in alveolar macrophages and contributes to the protection of the lung tissue. The main HO-1 inducers in the lungs are pro-inflammatory cytokines, such as TNF-α and IL-6, the heme group and nitric oxide (NO), as well as hypoxia and hyperoxia conditions [41] (Figure 1). There is sound evidence that states that HO-1 induction is a critical defense factor during acute and chronic lung processes [41][42][43].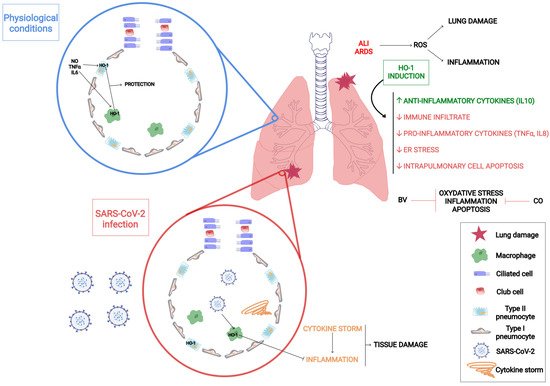
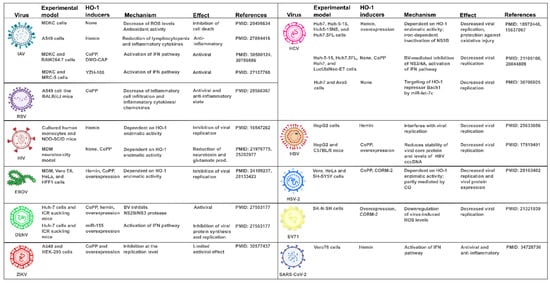
6. Unveiling How HO-1 Promotes Viral Clearance
7. HO-1 Induction as a Strategy against COVID-19
9. Conclusions
Hemin, a previously FDA and EMA approved drug for acute intermittent porphyria treatment, rises as a promising drug candidate, inducing HO-1 plasma concentration in humans, and posing a host defense advantage to fight SARS-CoV-2. Further work on optimal drug concentrations, pharmacokinetics and pharmacodynamics should be performed in order to prove hemin’s effectiveness (either alone or in combination with other drugs) to halt infection.References
- Marcella Prete; Elvira Favoino; Giacomo Catacchio; Vito Racanelli; Federico Perosa; SARS-CoV-2 Inflammatory Syndrome. Clinical Features and Rationale for Immunological Treatment. International Journal of Molecular Sciences 2020, 21, 3377, 10.3390/ijms21093377.
- Louise L. Dunn; Robyn G. Midwinter; Jun Ni; Hafizah A. Hamid; Christopher Parish; Roland Stocker; New Insights into Intracellular Locations and Functions of Heme Oxygenase-1. Antioxidants & Redox Signaling 2014, 20, 1723-1742, 10.1089/ars.2013.5675.
- Y. Naito; T. Takagi; T. Yoshikawa; Heme oxygenase-1: a new therapeutic target for inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 2004, 20, 177-184, 10.1111/j.1365-2036.2004.01992.x.
- Tomohisa Takagi; Yuji Naito; Katsura Mizushima; Yasuko Hirai; Akihito Harusato; Tetsuya Okayama; Kazhuhiro Katada; Kazuhiko Uchiyama; Osamu Handa; Takeshi Ishikawa; et al.Yoshito Itoh Heme oxygenase-1 prevents murine intestinal inflammation. Journal of Clinical Biochemistry and Nutrition 2017, 63, 169-174, 10.3164/jcbn.17-133.
- Ângelo Ferreira Chora; Paulo Fontoura; Andreia Cunha; Teresa Faria Pais; Sílvia Cardoso; Peggy P. Ho; Lowen Y. Lee; Raymond A. Sobel; Lawrence Steinman; Miguel P. Soares; et al. Heme oxygenase–1 and carbon monoxide suppress autoimmune neuroinflammation. Journal of Clinical Investigation 2007, 117, 438-447, 10.1172/jci28844.
- Ulrike Protzer; Stefan Seyfried; Maria Quasdorff; Gabriele Sass; Miriam Svorcova; Dennis Webb; Felix Bohne; Marianna Hösel; Peter Schirmacher; Gisa Tiegs; et al. Antiviral Activity and Hepatoprotection by Heme Oxygenase-1 in Hepatitis B Virus Infection. Gastroenterology 2007, 133, 1156-1165, 10.1053/j.gastro.2007.07.021.
- Janyra A. Espinoza; Pablo A. González; Alexis M. Kalergis; Modulation of Antiviral Immunity by Heme Oxygenase-1. The American Journal of Pathology 2017, 187, 487-493, 10.1016/j.ajpath.2016.11.011.
- Karl E. Anderson; Stephen Collins; Open-Label Study of Hemin for Acute Porphyria: Clinical Practice Implications. The American Journal of Medicine 2006, 119, 801.e1-801.e6, 10.1016/j.amjmed.2006.05.026.
- List of nationally authorised medicinal products . European Medicines Agency. Retrieved 2022-2-9
- Riccardo V. D'elia; Kate Harrison; Petra C. Oyston; Roman A. Lukaszewski; Graeme C. Clark; Targeting the “Cytokine Storm” for Therapeutic Benefit. Clinical and Vaccine Immunology 2013, 20, 319-327, 10.1128/cvi.00636-12.
- Linlin Chen; Huidan Deng; Hengmin Cui; Jing Fang; Zhicai Zuo; Junliang Deng; Yinglun Li; Xun Wang; Ling Zhao; Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204-7218, 10.18632/oncotarget.23208.
- Ruslan Medzhitov; Recognition of microorganisms and activation of the immune response. Nature Cell Biology 2007, 449, 819-826, 10.1038/nature06246.
- Cecilia L. Speyer; Peter A. Ward; Role of Endothelial Chemokines and Their Receptors during Inflammation. Journal of Investigative Surgery 2011, 24, 18-27, 10.3109/08941939.2010.521232.
- Lan Yang; Xueru Xie; Zikun Tu; Jinrong Fu; Damo Xu; Yufeng Zhou; The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduction and Targeted Therapy 2021, 6, 1-20, 10.1038/s41392-021-00679-0.
- Matthew Zirui Tay; Chek Meng Poh; Laurent Rénia; Paul A. Macary; Lisa F. P. Ng; The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology 2020, 20, 363-374, 10.1038/s41577-020-0311-8.
- Carolina Lucas; Patrick Wong; Jon Klein; Tiago B. R. Castro; Julio Silva; Maria Sundaram; Mallory K. Ellingson; Tianyang Mao; Ji Eun Oh; Benjamin Israelow; et al.Takehiro TakahashiMaria TokuyamaPeiwen LuArvind VenkataramanAnnsea ParkSubhasis MohantyHaowei WangAnne L. WyllieChantal B. F. VogelsRebecca EarnestSarah LapidusIsabel M. OttAdam J. MooreM. Catherine MuenkerJohn B. FournierMelissa CampbellCamila D. OdioArnau Casanovas-MassanaAbeer ObaidAlice Lu-CulliganAllison NelsonAnderson BritoAngela NunezAnjelica MartinAnnie WatkinsBertie GengChaney KalinichChristina HardenCodruta TodeasaCole JensenDaniel KimDavid McDonaldDenise ShepardEdward CourchaineElizabeth B. WhiteEric SongErin SilvaEriko KudoGiuseppe DeIuliisHarold RahmingHong-Jai ParkIrene MatosJessica NouwsJordan ValdezJoseph FauverJoseph LimKadi-Ann RoseKelly AnastasioKristina BrowerLaura GlickLokesh SharmaLorenzo SewananLynda KnaggsMaksym MinasyanMaria BatsuMary PetroneMaxine KuangMaura NakahataMelissa LinehanMichael H. AskenaseMichael SimonovMikhail SmolgovskyNicole SonnertNida NaushadPavithra VijayakumarRick MartinelloRupak DattaRyan HandokoSantos BermejoSarah ProphetSean BickertonSofia VelazquezTara AlpertTyler RiceWilliam Khoury-HanoldXiaohua PengYexin YangYiyun CaoYvette StrongRoy HerbstAlbert C. ShawRuslan MedzhitovWade L. SchulzNathan D. GrubaughCharles Dela CruzShelli FarhadianAlbert I. KoSaad B. OmerAkiko IwasakiYale Impact Team Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463-469, 10.1038/s41586-020-2588-y.
- Daniel Blanco-Melo; Benjamin E. Nilsson-Payant; Wen-Chun Liu; Skyler Uhl; Daisy Hoagland; Rasmus Møller; Tristan X. Jordan; Kohei Oishi; Maryline Panis; David Sachs; et al.Taia T. WangRobert E. SchwartzJean K. LimRandy A. AlbrechtBenjamin R. Tenoever Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036-1045.e9, 10.1016/j.cell.2020.04.026.
- Annsea Park; Akiko Iwasaki; Type I and Type III Interferons – Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host & Microbe 2020, 27, 870-878, 10.1016/j.chom.2020.05.008.
- Sandra Hervas-Stubbs; Jose Luis Perez-Gracia; Ana Rouzaut; Miguel F Sanmamed; Agnes Le Bon; Ignacio Melero; Direct Effects of Type I Interferons on Cells of the Immune System. Clinical Cancer Research 2011, 17, 2619-2627, 10.1158/1078-0432.ccr-10-1114.
- David E. Levy; The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine & Growth Factor Reviews 2001, 12, 143-156, 10.1016/s1359-6101(00)00027-7.
- S. Goodbourn; L. Didcock; R. E. Randall; Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. Journal of General Virology 2000, 81, 2341-2364, 10.1099/0022-1317-81-10-2341.
- Colleen Cebulla; Daniel M. Miller; Daniel D. Sedmak; Viral Inhibition of Interferon Signal Transduction. Intervirology 1998, 42, 325-330, 10.1159/000053968.
- Sanjay Kumar; Uday Bandyopadhyay; Free heme toxicity and its detoxification systems in human. Toxicology Letters 2005, 157, 175-188, 10.1016/j.toxlet.2005.03.004.
- Shunsuke Hayashi; Yoshiaki Omata; Hiroshi Sakamoto; Yuichiro Higashimoto; Takayuki Hara; Yasuhiro Sagara; Masato Noguchi; Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 2004, 336, 241-250, 10.1016/j.gene.2004.04.002.
- Akihiro Yachie; Yo Niida; Taizo Wada; Noboru Igarashi; Hisashi Kaneda; Tomoko Toma; Kazuhide Ohta; Yoshihito Kasahara; Shoichi Koizumi; Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. Journal of Clinical Investigation 1998, 103, 129-135, 10.1172/jci4165.
- Yunlong Li; Kuai Ma; Zhongyu Han; Mingxuan Chi; Xiyalatu Sai; Ping Zhu; Zhaolun Ding; Linjiang Song; Chi Liu; Immunomodulatory Effects of Heme Oxygenase-1 in Kidney Disease. Frontiers in Medicine 2021, 8, -, 10.3389/fmed.2021.708453.
- Kun-Chun Chiang; Kang-Shuo Chang; Shu-Yuan Hsu; Hsin-Ching Sung; Tsui-Hsia Feng; Mei Chao; Horng-Heng Juang; Human Heme Oxygenase-1 Induced by Interleukin-6 via JAK/STAT3 Pathways Is a Tumor Suppressor Gene in Hepatoma Cells. Antioxidants 2020, 9, 251, 10.3390/antiox9030251.
- Geraldine Gueron; Adriana De Siervi; Mercedes Ferrando; Marcelo Salierno; Paola De Luca; Belen Elguero; Roberto Meiss; Nora Navone; Elba Vazquez; Critical Role of Endogenous Heme Oxygenase 1 as a Tuner of the Invasive Potential of Prostate Cancer Cells. Molecular Cancer Research 2009, 7, 1745-1755, 10.1158/1541-7786.mcr-08-0325.
- S Immenschuh; H Schröder; Heme oxygenase-1 and cardiovascular disease. Histol. Histopathol. 2006, 21, 679-685, 10.14670/HH-21.679.
- Xiaoliang Lin; Jiajia Lv; Dandan Ge; Haitao Bai; Yungang Yang; Jinzhun Wu; Heme oxygenase‐1 alleviates eosinophilic inflammation by inhibiting STAT3‐SOCS3 signaling. Pediatric Pulmonology 2020, 55, 1440-1447, 10.1002/ppul.24759.
- Shehzad Z. Sheikh; Refaat A. Hegazi; Taku Kobayashi; Joseph C. Onyiah; Steven M. Russo; Katsuyoshi Matsuoka; Antonia R. Sepulveda; Fengling Li; Leo E. Otterbein; Scott E. Plevy; et al. An Anti-Inflammatory Role for Carbon Monoxide and Heme Oxygenase-1 in Chronic Th2-Mediated Murine Colitis. The Journal of Immunology 2011, 186, 5506-5513, 10.4049/jimmunol.1002433.
- Ananta Paine; Britta Eiz-Vesper; Rainer Blasczyk; Stephan Immenschuh; Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology 2010, 80, 1895-1903, 10.1016/j.bcp.2010.07.014.
- Sotiria Tzima; Panayiotis Victoratos; Ksanthi Kranidioti; Maria Alexiou; George Kollias; Myeloid heme oxygenase–1 regulates innate immunity and autoimmunity by modulating IFN-β production. Journal of Experimental Medicine 2009, 206, 1167-1179, 10.1084/jem.20081582.
- Katja Kotsch; Paulo N.A. Martins; Roman Klemz; Uwe Janssen; Bernhard Gerstmayer; Annelie Dernier; Anja Reutzel-Selke; Ulrike Kuckelkorn; Stefan G. Tullius; Hans-Dieter Volk; et al. Heme Oxygenase-1 Ameliorates Ischemia/Reperfusion Injury by Targeting Dendritic Cell Maturation and Migration. Antioxidants & Redox Signaling 2007, 9, 2049-2064, 10.1089/ars.2007.1801.
- Christine Chauveau; Séverine Rémy; Pierre Joseph Royer; Marcelo Hill; Séverine Tanguy-Royer; François-Xavier Hubert; Laurent Tesson; Régis Brion; Gaëlle Beriou; Marc Grégoire; et al.Régis JosienMaria Cristina CuturiIgnacio Anegon Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 2005, 106, 1694-1702, 10.1182/blood-2005-02-0494.
- Matthias H. Kapturczak; Clive Wasserfall; Todd Brusko; Martha Campbell-Thompson; Tamir M. Ellis; Mark A. Atkinson; Anupam Agarwal; Heme Oxygenase-1 Modulates Early Inflammatory Responses: Evidence from the Heme Oxygenase-1-Deficient Mouse. The American Journal of Pathology 2004, 165, 1045-1053, 10.1016/s0002-9440(10)63365-2.
- Leo E. Otterbein; Fritz H. Bach; Jawed Alam; Miguel Soares; Hong Tao Lu; Mark Allen Wysk; Roger J. Davis; Richard A. Flavell; Augustine M. K. Choi; Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature Medicine 2000, 6, 422-428, 10.1038/74680.
- Tzong-Shyuan Lee; Lee-Young Chau; Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature Medicine 2002, 8, 240-246, 10.1038/nm0302-240.
- Miguel P. Soares; Mark P. Seldon; Isabel Pombo Gregoire; Tatiana Vassilevskaia; Pascal O. Berberat; Jia Yu; Tung-Yu Tsui; Fritz H. Bach; Heme Oxygenase-1 Modulates the Expression of Adhesion Molecules Associated with Endothelial Cell Activation. The Journal of Immunology 2004, 172, 3553-3563, 10.4049/jimmunol.172.6.3553.
- Toshitaka Nakamura; Isao Naguro; Hidenori Ichijo; Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochimica et Biophysica Acta (BBA) - General Subjects 2019, 1863, 1398-1409, 10.1016/j.bbagen.2019.06.010.
- Laura E. Fredenburgh; Mark A. Perrella; S. Alex Mitsialis; The Role of Heme Oxygenase-1 in Pulmonary Disease. American Journal of Respiratory Cell and Molecular Biology 2007, 36, 158-165, 10.1165/rcmb.2006-0331tr.
- Dirk-Jan Slebos; Stefan W Ryter; Augustine M K Choi; Heme oxygenase-1 and carbon monoxide in pulmonary medicine. Respiratory Research 2003, 4, 7-7, 10.1186/1465-9921-4-7.
- Caterina Di Pietro; Hasan H. Öz; Thomas S. Murray; Emanuela M. Bruscia; Targeting the Heme Oxygenase 1/Carbon Monoxide Pathway to Resolve Lung Hyper-Inflammation and Restore a Regulated Immune Response in Cystic Fibrosis. Frontiers in Pharmacology 2020, 11, 1059, 10.3389/fphar.2020.01059.
- B. Müller; Hans-Georg Kräusslich; Antiviral Strategies. Diabetes - Perspectives in Drug Therapy 2008, 189, 1-24, 10.1007/978-3-540-79086-0_1.
- John Kenneth Baillie; Paul Digard; Influenza — Time to Target the Host?. New England Journal of Medicine 2013, 369, 191-193, 10.1056/nejmcibr1304414.
- Mack Sheraton; Neha Deo; Rahul Kashyap; Salim Surani; A Review of Neurological Complications of COVID-19. Cureus 2020, 12, e8192, 10.7759/cureus.8192.
- Rose H. Manjili; Melika Zarei; Mehran Habibi; Masoud H. Manjili; COVID-19 as an Acute Inflammatory Disease. The Journal of Immunology 2020, 205, 12-19, 10.4049/jimmunol.2000413.
- Neelu Batra; Cristabelle De Souza; Jyoti Batra; Alan Raetz; Ai-Ming Yu; The HMOX1 Pathway as a Promising Target for the Treatment and Prevention of SARS-CoV-2 of 2019 (COVID-19). International Journal of Molecular Sciences 2020, 21, 6412, 10.3390/ijms21176412.
- Yonghua Zhu; Yunjuan Sun; Kunlin Jin; David A. Greenberg; Hemin induces neuroglobin expression in neural cells. Blood 2002, 100, 2494-2498, 10.1182/blood-2002-01-0280.
- Hui‑Min Li; Ying‑Lu Shi; Di Wen; Huan‑Min Luo; Xi Lin; Fei Xiao; A novel effective chemical hemin for the treatment of acute carbon monoxide poisoning in mice. Experimental and Therapeutic Medicine 2017, 14, 5186-5192, 10.3892/etm.2017.5157.
- Xiangping Lu; Jing Chen-Roetling; Raymond F. Regan; Systemic hemin therapy attenuates blood–brain barrier disruption after intracerebral hemorrhage. Neurobiology of Disease 2014, 70, 245-251, 10.1016/j.nbd.2014.06.005.
- David Olagnier; Ensieh Farahani; Jacob Thyrsted; Julia Blay-Cadanet; Angela Herengt; Manja Idorn; Alon Hait; Bruno Hernaez; Alice Knudsen; Marie Beck Iversen; et al.Mirjam SchillingSofie E. JørgensenMichelle ThomsenLine S. ReinertMichael LappeHuy-Dung HoangVictoria H. GilchristAnne Louise HansenRasmus OttosenCamilla G. NielsenCharlotte MøllerDemi van der HorstSuraj PeriSiddharth BalachandranJinrong HuangMartin JakobsenEsben B. SvenningsenThomas B. PoulsenLydia BartschAnne L. ThielkeYonglun LuoTommy AlainJan RehwinkelAntonio AlcamíJohn HiscottTrine H. MogensenSøren R. PaludanChristian K. Holm SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nature Communications 2020, 11, 1-12, 10.1038/s41467-020-18764-3.
- Antonio Cuadrado; Marta Pajares; Cristina Benito; José Jiménez-Villegas; Maribel Escoll; Raquel Fernández-Ginés; Angel J. Garcia Yagüe; Diego Lastra; Gina Manda; Ana I. Rojo; et al.Albena T. Dinkova-Kostova Can Activation of NRF2 Be a Strategy against COVID-19?. Trends in Pharmacological Sciences 2020, 41, 598-610, 10.1016/j.tips.2020.07.003.
