When facing an acute viral infection, our immune systems need to function with finite precision to enable the elimination of the pathogen, whilst protecting our bodies from immune-related damage. In many instances however this ‘perfect balance’ is not achieved, factors such as ageing, cancer, autoimmunity and cardiovascular disease all skew the immune response which is then further distorted by viral infection. SARS-CoV-2 infection skews the immune response towards an overwhelmingly inflammatory phenotype. Restoration of NK cell effector functions has the potential to correct the delicate immune balance required to effectively overcome SARS-CoV-2 infection.
- Natural Killer Cells
- COVID-19
- SARS-CoV-2
1. Introduction
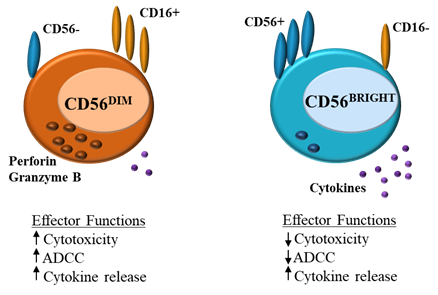
Natural killer (NK) cells form part of the innate immune system, where they serve as a first-line defense against acute infection and cancer, whilst also regulating the adaptive immune response [1]. NK cell function is tightly regulated by a balance of activating and inhibitory germline-encoded receptors. NK cell activation results in cytotoxic degranulation and the production of inflammatory cytokines, killing target cells [21][12][3][4]. NK cells can be divided into CD56DIM and CD56BRIGHT subsets (Figure 1). CD56DIMCD16+ NK cells are abundant in the blood and are cytotoxic, expressing perforin and producing IFN-γ, CD56BRIGHTCD16− cells on the other hand are found in lymphoid tissues, these cells lack perforin activity and instead produce cytokines such as IFN-γ in response to stimulation with IL-12, IL-15 and IL-18, increasing NK effector function [54][43][65].
Figure 1. Natural killer (NK) cells: differences in effector function between CD56DIM and CD56BRIGHT
(ADCC = antibody-dependent cell cytotoxicity).
2. Functions of NK cells
Healthy cells express major histocompatibility complex class I (MHC I) molecules which mark these cells as ‘self’, MHC I act as ligands for inhibitory receptors on NK cells and contribute to the ‘self-tolerance’, by preventing NK-cell-killing of these cells [2,3][1][2]. The MHC I–specific inhibitory receptors include the killer cell immunoglobulin-like receptors (KIRs) (KLRG1, and TIGIT) and the lectin-like CD94-NKG2A heterodimers [21][54]. Cellular stress, impaired KIR engagement and MHC 1 downregulation, associated with infection or cancer growth, lower the inhibitory signalling threshold, resulting in NK cell activity receptor upregulation [21][43][54]. NK cells express numerous activating receptors, which in response to infection or cellular distress, induce signalling pathways (NKG2D, CD244, NKp30, NKp46) that trigger NK cell responses [1][2][3][46][7]. Through co-activation these receptors overcome the NK regulatory balance to mount an effective response [21][76]. Activated NK cells induce killing through multiple mechanisms; (1) NK cell activation can result in direct lysis of target cells, through cytotoxic degranulation by perforin and granzymeB, (2) indirect elimination of target cells through the production of inflammatory cytokines, such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), (3) NK cells express CD16, which allows for the detection of antibody-coated target cells, leading to NK cell antibody-dependent cell cytotoxicity (ADCC) and (4) through interaction with accessory cells such as monocytes, NK cells may indirectly also interact with infectious ‘nonself’ and Toll-like receptor (TLR) ligands, inducing IFNγ production and enhancing cytotoxicity [1][23][4][87]. NK function can also be downregulated, this is achieved through ligand interaction with inhibitory receptors such as killer immunoglobulin-like receptors (KIRs) and the C-type lectin-like receptor CD94-NKG2A which suppress NK cell activation [1][2].
Apart from their critical role in pathogen elimination, an equally important role of NK cells is their potential to limit the immune response, specifically T cells. Indirectly this is achieved through the modulation of antigen presenting cells (e.g., dendritic cells (DCs)) and directly through interactions with the T cells themselves [1][2]. During acute infection, NK cells can promote the differentiation of naïve CD4+ T cells into Th1 T cells through the secretion of IFNγ, thereby leading to increased pathogen control, they can also decrease T cell priming through IL-10 [1][2]. NK cell promotion of mature DCs leads to increased antigen presentation and thus an increased cytotoxic (CD8+) T cell response [1][23][4]. Otherwise, NK cells also regulate the adaptive immune response through cytotoxic killing of activated T cells. Activated T cells often express higher levels of activating ligands (NKG2D) and decreased inhibitory ligands (MHC I), resulting in their recognition and elimination [21][32]. Resultant reduction in T follicular helper (Tfh) responses hampers the development of the B cell response, as Tfh are responsible for driving B cell differentiation into memory B cells and plasma cells that produce antibodies [98].
3. Prospect
When facing an acute viral infection, our immune systems need to function with finite precision to enable the elimination of the pathogen, whilst protecting our bodies from immune-related damage. In many instances however this ‘perfect balance’ is not achieved, factors such as ageing, cancer, autoimmunity and cardiovascular disease all skew the immune response which is then further distorted by the viral infection. In SARS-CoV-2 infection, although the vast majority of COVID-19 cases are mild, over 890,000 (as of 8 September 2020) people have died [109]. Many of these deaths are associated with a severe inflammatory cytokine release, resulting in extreme clinical manifestations such as acute respiratory distress syndrome (ARDS) and hemophagocytic lymphohistiocytosis (HLH) [10][11][12][13]. Severe complications are more common in elderly patients and patients with cardiovascular diseases [12][13][14]. Natural killer (NK) cells play a critical role in modulating the immune response and in both of these patient groups NK cell effector functions are blunted [14][15][16].
In viral infection, defective cytotoxicity leads to the accumulation of antigenic stimuli, perpetuating inflammation and in that triggering tissue damage. Preliminary studies in COVID-19 patients with severe disease suggests a reduction in NK cell number and function, and elevated exhaustion marker levels [16][17][18], resulting in decreased cytotoxicity and thus reduced clearance of infected and activated cells. Patients also suffer unchecked elevation of tissue-damaging inflammatory markers, many of which further diminish NK cell effector functions [1312][18][19][20]. Combined, these factors indicate that diminished NK cell cytotoxicity and immune regulation lead to a critical inflammatory phenotype in SARS-CoV-2 infection. Restoration of NK cell effector functions has the potential to correct the delicate immune balance required to effectively overcome SARS-CoV-2 infection, reducing morbidity and mortality in COVID-19 patients [2120].
Natural killer cells play a central role in maintaining immune homeostasis, a critical requirement when facing the challenge of a novel pathogen. SARS-CoV-2 infection has been shown to impede NK cell function, thus disrupting this vital balance. With factors such as ageing and other comorbidities, leading to further skewing, the current trials investigating drugs and biologicals, which improve NK cell function, may prove to be monumental in our fight against COVID-19.
References
- Schuster, I.S.; Coudert, J.D.; Andoniou, C.E; Degli-Esposti, M.A.; "Natural Regulators": NK cells as modulators of T cell immunity. FronLanier, L.L.; Up on a tightrope: natural killer cell activation and inhibition.. Nat Immunol 20016, 7, 235.8, 9, 495-502.
- Lanier, L.L.; Up on a tightrope: natural killer cell activation and inhibition.. NKarre, K.; NK Cells, MHC class 1 molecules and the missing self. Scatnd J Immunol 2008, 9, 495-502.2, 55, 221-228.
- Karre, K.; NK Cells, MHC class 1 molecules and the missing self. ScVivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S.; Functions of natural killer cells. Nand Jt Immunol 2002, 55, 221-228.8, 9, 503-510.
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S.; Functions of natural killer cells. NatLong, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S.; Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2008, 9, 503-510.13, 31, 227-258.
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S.; Controlling natural killer cell responses: integration of signals for activation and inhibition. ACooper, M.A.; Fehniger, T.A.; Caligiuri, M.A.; The biology of human natural killer-cell subsets. Trennuds Rev Immunol 20013, 31, 227-258., 22, 633-640.
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A.; The biology of human natural killer-cell subsets. TrenBryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O.; Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Bloods Immunol 2001, 22, 633-640.6, 107, 159-166.
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O.; Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. BChan, C.J.; Smyth, M.J.; Martinet, L.; Molecular mechanisms of natural killer cell activation in response to cellular stress. Cellood Death Differ 2006, 14, 2107, 159-166., 5-14.
- Chan, C.J.; Smyth, M.J.; Martinet, L.; Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell DeatZhang, X.; Ing, S.; Fraser, A.; Chen, M.; Khan, o.; Zakem, J.; Davis, W.; Quinet, R.; Follicular helper T cells: new insights into mechanisms of autoimmune diseases. Osch Diffsner J 2014, 23, 1, 5-14.3, 131-9.
- Zhang, X.; Ing, S.; Fraser, A.; Chen, M.; Khan, o.; Zakem, J.; Davis, W.; Quinet, R.; Follicular helper T cells: new insights into mechanisms of autoimmune diseases. Oschsner J 2013, 13, 131-9.Coronavirus disease (COVID-19) pandemic . World Health Organization. Retrieved 2020-9-9
- Coronavirus disease (COVID-19) pandemic . World Health Organization. Retrieved 2020-9-9Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH across speciality collaboration, U.K., COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033-1034.
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH across speciality collaboration, U.K., COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al.Zhao, Y.Li, Y.Wang, X.Peng, Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, , 32395, 1033-1034., 1061-1069.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al.Zhao, Y.Li, Y.Wang, X.Peng, Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al.Cheng, Z.Yu, T.Xia, J.Wei, Y.Wu, W.Xie, X.Yin, W.Li, H.Liu, M.Xiao, Y.Gao, H.Guo, L.Xie, J.Wang, G.Jiang, R.Gao, Z.Jin, Q.Wang, J.Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 323, 1061-1069.95, 497-506.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al.Cheng, Z.Yu, T.Xia, J.Wei, Y.Wu, W.Xie, X.Yin, W.Li, H.Liu, M.Xiao, Y.Gao, H.Guo, L.Xie, J.Wang, G.Jiang, R.Gao, Z.Jin, Q.Wang, J.Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. LaWu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al.Zhang, Y.Song, J.Wang, S.Chao, Y.Yang, Z.Xu, J.Zhou, X.Chen, D.Xiong, W.Xu, L.Zhou, F.Jiang, J.Bai, C.Zheng, J.Song, Y., Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internc Met d 2020, 395, 497-506., 180, 1-11.
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al.Zhang, Y.Song, J.Wang, S.Chao, Y.Yang, Z.Xu, J.Zhou, X.Chen, D.Xiong, W.Xu, L.Zhou, F.Jiang, J.Bai, C.Zheng, J.Song, Y., Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JPonnappan, S.; Ponnappan, U.; Aging and immune function: molecular mechanisms to interventions. AMA Intern Mioxid Red ox Signal 2020, 11, 180, 1-11.4, 1551-1585.
- Ponnappan, S.; Ponnappan, U.; Aging and immune function: molecular mechanisms to interventions. ABonaccorsi, I.; Spinelli, D.; Cantoni, C.; Barilla, C.; Pipito, N.; De Pasquale, C.; Oliveri, D.; Cavaliere, R.; Carrega, P.; Benedetto, F.; et al.Ferlazzo, G. Symptomatic Carotid Atherosclerotic Plaques Are Associated With Increased Infiltration of Natural Killer (NK) Cells and Higher Serum Levels of NK Activating Receptor Ligands. Frontioxid Redox SignaImmunol 2011, 9, 14, 1551-1585.0, 1503.
- Bonaccorsi, I.; Spinelli, D.; Cantoni, C.; Barilla, C.; Pipito, N.; De Pasquale, C.; Oliveri, D.; Cavaliere, R.; Carrega, P.; Benedetto, F.; et al.Ferlazzo, G. Symptomatic Carotid Atherosclerotic Plaques Are Associated With Increased Infiltration of Natural Killer (NK) Cells and Higher Serum Levels of NK Activating Receptor Ligands. FrZheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z.; Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Montl Immunol 202019, , 10, 1503.7, 533-535.
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z.; Functional exhaustion of antiviral lymphocytes in COVID-19 patients. CeLi, D.; Chen, Y.; Liu, H.; Jia, Y.; Li, F.; Wang, W.; Wu, J.; Wan, Z.; Cao, Y.; Zeng, R.; et al. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study.. Signall Transduct Mol Immunol Target Ther 2020, 17, 533-535., 5, 62.
- Li, D.; Chen, Y.; Liu, H.; Jia, Y.; Li, F.; Wang, W.; Wu, J.; Wan, Z.; Cao, Y.; Zeng, R.; et al. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study.. SQin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al.Tian, D. S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clignal Transduct Target Ther Infect Dis 2020, 5, 62., 71, 762-768.
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al.Tian, D. S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. ClOsman, M. S.; van Eeden, C.; Tervaert, J. W. C.; Fatal COVID-19 infections: Is NK cell dysfunction a link with autoimmune HLH. Autoimmun Infect Dis Rev 2020, 7, 1, 762-768.9, 102561.
- Osman, M. S.; van Eeden, C.; Tervaert, J. W. C.; Fatal COVID-19 infections: Is NK cell dysfunction a link with autoimmune HLH. Autoimmun Rev van Eeden, C.; Khan, L.; Osman, M.S.; Tervaert, J.W.C.; Natural Killer Cell Dysfunction and its Role in COVID-19. IMJS 2020, , 219, 102561., 6351.
- van Eeden, C.; Khan, L.; Osman, M.S.; Tervaert, J.W.C.; Natural Killer Cell Dysfunction and its Role in COVID-19. IMJS 2020, 21, 6351.