Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sevilay Tümkaya Yılmaz and Version 2 by Amina Yu.
Chronic pain in cancer survivors is related to obesity, malnutrition, nutritional deficiency, diet quality, immune system, systemic inflammation, and gut microbiota. As seen clearly, dietary interventions may provide weight reduction, a healthy body weight, good diet quality, regulations in systemic inflammation and immune system, and a healthy gut microbiota environment that could modify aforementioned pain-related pathways/mechanisms. For that reason, nutrition might have the potential to transition from being only prevention for cancer recurrence or cancer itself to a modality for chronic pain management for cancer survivors.
- cancer survivors
- chronic pain
- pain management
- nutrition
- diet
1. Pain and Nutrition in Cancer Survivors: An Update from Cancer and Chronic Pain Literature
Since both nutritional and chronic pain mechanisms and pathways are known for their complexity, it does not come as a surprise that research covering the link between both is complex, ambiguous and involves different explanatory components[1] [24]. Dietary factors (like food preparation, food processing and dietary patterns) exert their impact by several pathways and mechanisms such as glucose-insulin homeostasis, blood lipids, blood pressure, functions of the endothelial, cardiac and adipocyte systems, the gut microbiome, systemic inflammation, and hunger and satiety[2] [28]. Chronic pain and its treatment (like opioids), in turn, are known to have an interplay with the nervous systems and the immune system[3][4] [29,30]. This narrative piece shines a light on the following different pathways and mechanisms to link diet/nutrition and (chronic) pain in cancer survivors (Figure 1): (1) through obesity; (2) through malnutrition, nutritional deficiency, and diet quality; (3) through the immune system and systemic inflammation; and (4) through gut microbiota.
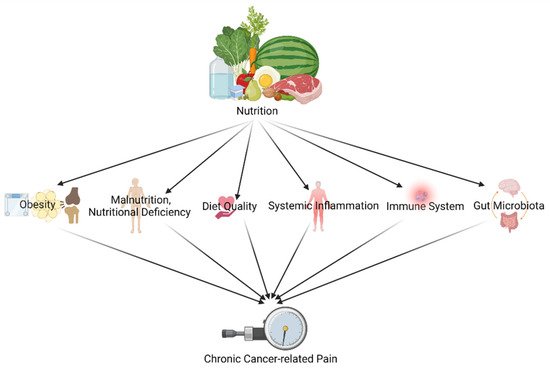
Figure 1. Different pathways and mechanisms to link diet/nutrition and (chronic) pain in cancer survivors (created with BioRender.com accessed on 23 December 2021).
2. Impact of Diet and Nutrition on Pain in Cancer Survivors through Obesity
Obesity continues to be a major public health concern, and it is especially frequent among cancer patients, thus determining its long-term impact on the expanding population of cancer survivors is critical[5] [31]. Moreover, it has been known since the 1970s that women with breast cancer receiving adjuvant chemotherapy experience weight gain, commonly reported as 2–5 kg but with great variance [6][32]. Obesity, defined as a BMI of 30 kg/m2 or higher, is also a common risk factor for poor health-related quality of life in cancer survivors, in particular colorectal, breast, and prostate cancer survivors[7] [33].
Obesity can cause chronic pain through two primary processes: mechanical stress, which occurs when extra body weight puts stress on joints in the musculoskeletal system, and systemic proinflammatory state, which is linked to adipose tissue and can increase pain[8] [34]. Obesity (particularly caused by excessive abdominal fat) is related to an increment in chronic systemic inflammation which can have a contribution to central sensitization [9][35]. According to Emery et al., a diet rich in anti-inflammatory foods, such as the consumption of seafood and plant protein, appears to be linked to the relationship between body fat and pain ratings in healthy adults so they advocated that diet can be addressed as part of pain treatment and evaluation, specifically among overweight and obese people[10] [36]. Additionally, obesity has been linked to microbial homeostasis distortion, with a decrease in bacterial biodiversity and altered expression of bacterial genes, particularly those involved in dietary energy extraction[11] [37]. The increased understanding of the interactions between the gut microbiota and the central nervous system, also known as the gut-brain axis, makes the hypothesis of the gut microbiota’s possible effect on the pain processing and the pain perception reasonable[12] [38], so does obesity. With good-to-moderate patient-centered evidence, the most recent review (n = 26) in taxane and platinum-treated cancer patients found a link between obesity and increased severity or occurrence of chemotherapy-induced peripheral neuropathy (CIPN)[13] [39]. Additionally, weight gain (>5%) following breast cancer was found to be positively associated with above-average pain[14] [40].
However, evidence on the relationship between obesity and (chronic) pain in cancer survivors remains very limited. One study found a correlation between a higher BMI and a lower physical quality of life in cancer survivors, including more pain even after taking into consideration age, race, education level, cancer type, and comorbidities[15] [41]. Again, in a meta-analysis conducted by Leysen et al.[16] [4], among other factors, a BMI of 30 kg/m2 or higher was substantially linked with the development of chronic pain in breast cancer survivors. In another study, it has been suggested that cancer survivors with CIPN and co-occurring obesity may be more at risk of lower quality of life due to higher symptom severity and pain than non-obese survivors[7] [33]. In parallel, among cancer survivors with CIPN who received platinum and/or taxane chemotherapeutic compounds, overweight and obese survivors experienced more severe pain and higher pain interference scores than normal-weight survivors [17][42]. Similar to that overweight or obese breast cancer survivors with weight loss of ≥ 5% showed improvement in their pain at 12 months, but these changes were not significantly different from those who lost < 5%[18] [43]. Therefore, weight reduction techniques for obese cancer survivors suffering from chronic pain could be a key factor within pain management for this population. Moreover that studies examining whether dietary management results in pain relief in cancer patients receiving chemotherapy or in survivors after treatment (or in any other cancer treatment associated with pain) are urgently needed and represent an important research priority.
3. Impact of Diet and Nutrition on Pain in Cancer Survivors through Malnutrition, Nutritional Deficiency, and Diet Quality
Cancer patients suffer from a large catabolic imbalance which causes weight loss, the key indicator of cancer-associated malnutrition[19] [44]. The prevalence of malnutrition is estimated to be between 50 and 80%, depending on the tools used and the populations studied[20] [45], and can reach up to 85% of patients with certain cancers such as pancreatic[21] [46]. According to many proposed mechanisms, which varying from signaling molecules included within the diet (such as oxidized lipids), vagus nerve activation, microbiota alterations, and oxidative stress to maladaptive neuroplasticity induced by hyper-palatable energy-dense foods, poor nutrition may also cause activation of the immune system, in particular by glial activation with increased inflammation and nervous system hypersensitivity as a consequence[22] [47]. Available data clearly revealed that well-nourished breast cancer survivors had improved functions and less symptoms including pain in comparison to malnourished breast cancer survivors[23] [48].
Additionally, nutritional reduction is frequently acknowledged as a component of the cancer course and treatment[24] [49]. During chemotherapy, compared to women without cancer, breast cancer patients reported a significantly lower absolute protein, fat, and alcohol intake, but not carbohydrates and fiber. [25][50]. In Iranian breast cancer survivors, the average daily energy intake was lower than the estimated energy requirement as a reference value, with just 34% of participants meeting the estimated energy requirement, whereas the mean intakes of vitamin D, vitamin E, iron, and magnesium were insufficient to meet the Food and Nutrition Board’s (1997–2001) guideline of dietary reference intakes [23][48].
In cancer patients, both at the time of diagnosis and during treatment, micronutrient deficits are common [26][11]. For instance, pancreatic cancer patients who have had their pancreas removed are at risk of many nutrition-related comorbidities, including an impact on gastrointestinal and hepatic function, glycaemic regulation, bone health, and the status of many micronutrients such as vitamin A, B12, D, E, iron, magnesium and zinc[27] [51]. Similarly, one possible metabolic consequence after a gastrectomy after gastric cancer is vitamin B12 deficiency, which may lower cancer survivors’ quality of life[28] [52].
Importantly, low macro/micronutrient consumption, particularly omega-3 fatty acids, vitamins B1, B3, B6, B12, and D, magnesium, zinc, and -carotene, is associated with chronic neuropathic or inflammatory pain[29] [53]. As seen in multiple systematic studies on various pain conditions, including aromatase inhibitor(AI)-related arthralgia in breast cancer [30][54], supplementing the diet with these specific nutrients helps to alleviate chronic pain [31][55]. For example, since estrogen increases vitamin D receptor activation, a low estrogen status could potentially reduce the available active vitamin D amount; 75 to 90% of women receiving AI therapy have a vitamin D deficiency [32][56], which might negatively contribute to a chronic pain state [33][57]. It is known that vitamin D deficiency causes a muscle and joint aches syndrome similar to Aromatase Inhibitor-Induced Arthralgias (AIA) [32][56]. As a result, it is suggested that vitamin D can have a crucial role in several cellular activities considered preventive against the development and modulation of chronic pain [33][57].
Interestingly, cancer and its treatment may increase the requirement for antioxidant nutrient intake such as vitamin C because of the increased free radicals[26] [11]. Administrating some anti-cancer therapies has shown a significant reduction in patients’ vitamin C concentrations and report of scurvy (vitamin C deficiency disease)-like symptoms so cancer patients are one of the many patient groups who have a high prevalence of hypovitaminosis C and vitamin C deficiency [34][58]. IThe mini-review that was aboutreviewed the few current trials exploring the impact of IV vitamin C on cancer- and chemotherapy-related quality of life discovered considerable reductions in pain following vitamin C administration [35][59].
Additionally, it is known that magnesium supplementation is used in a variety of neuropathic pain situations, including cancer-related neuropathic pain and chemotherapy-related neuropathy, as is shown in ta recent review that looked for nutritional supplements for the treatment of neuropathic pain [36][60]. Still, evidence supporting magnesium supplementation for the treatment of (neuropathic pain) following cancer is lacking.
Similarly, short-chain fatty acids (SCFAs) are essential mediators of pain since they fundamentally modulate inflammation[36] [24]. In a network meta-analysis with randomized controlled trials included in the six systematic reviews, Kim et al. [30][54] showed that omega-3 fatty acids are one of the treatment modalities which attained significant improvement in pain severity compared to wait list controls in breast cancer survivors with AIA. However, since the overall confidence level of each review was limited, no recommendations can be made at present to reduce pain in patients with AIA [30][54].
Apart from this, several studies have found a link between cancer survivors’ health-related quality of life and their adherence to general non-cancer-specific dietary guidelines, such as the Healthy Eating Index and the Mediterranean diet[15][21] [41,61]. Higher adherence to the traditional Mediterranean Diet (high consumption of plant-based foods (vegetables, fruit, whole grains, legumes, nuts, olive oil) and low or limited consumption of red meat, milk, and sweets) were linked to higher physical functioning and health status, as well as lower pain and insomnia symptoms, suggesting that this diet may play a role in the quality of life of recently diagnosed female breast cancer patients [37][62]. Likewise, Wayne et al.[38] [63] claimed that women newly diagnosed with first primary breast cancer (in situ or stage I to IIIA disease) with excellent diet quality according to the Diet Quality Index received higher quality of life scores than women with poor diet quality, including physical health subscale category “bodily pain” with the highest scores.
Furthermore, cancer survivors are advised to follow some diet recommendations from the American Cancer Society (ACS) Guideline on Diet and Physical Activity for Cancer Prevention and the World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) Cancer Prevention. Higher diet scores were associated with many aspects, including bodily pain among breast cancer survivors with stage II–III cancer, according to a cross-sectional study that looked at whether adherence to the American Cancer Society (ACS) guidelines was associated with health-related quality of life (HRQoL) among Korean breast cancer survivors [39][64]. A study of Chinese patients with breast cancer who followed the WCRF/AICR guidelines (BMI, physical activity, and diet) before and after their diagnosis found that following the BMI prescription resulted in reduced pain scores while adherence to dietary recommendations, on the other hand, was not linked to pain scores [40][65].
4. Impact of Diet and Nutrition on Pain in Cancer Survivors through the Immune System and Systemic Inflammation
Chronic pain frequently arises from a permanent pro-inflammatory state [31][55]. The proposed pathophysiology and mechanisms that maintain chronic pain emerge constantly, yet as part of the maladaptive synaptic plasticity related to chronic pain, proposing permanent low-grade inflammation (neuroinflammation) as a primary driver makes therapeutic approaches targeting immune activation to reduce the pro-inflammatory state important to consider for chronic pain management [22][47].
This pro-inflammatory state is also a characteristic of cancer. Independent of the increment in neural density noticed in the tumor environment, numerous pain modulating agents such as hydrogen ions, tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), prostaglandins, interleukin-1 (IL-1) and IL-6 are set free into the tumor vicinity, sensitizing and stimulating sensory fibers, possibly contributing to neuronal hyperexcitability and pain [41][1].
Dietary components have the potential to have substantial inflammatory or anti-inflammatory features[42] [68]. Inflammation is linked to dietary consumption of omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) in healthy populations, according to observational studies since higher omega-3 PUFAs are associated with lower levels of pro-inflammatory indicators such as interleukin (IL)-6, IL-1 receptor antagonist, TNF-α, and C-reactive protein (CRP), as well as higher levels of anti-inflammatory indicators such as IL-10 and transforming growth factor β [43][69]. Additionally, it is known that within the neurological system, certain combinations of omega-3 and micronutrients (like vitamin A and D) may show an even bigger synergistic effect on inhibiting microglial-mediated neuroinflammation [44][70]. Similarly, high consumption of dietary fibers is inversely related to the circulating inflammatory markers interleukin 6 (IL-6) and tumor necrosis factor α receptor 2 (TNF-α-R2) in postmenopausal women and C-reactive protein (CRP) in breast cancer survivors [45][71]. Moreover, a diet high in fruit, vegetables, whole grains, white meat, tomato, legumes, tea, and fruit juices is substantially and inversely related to indicators of systemic inflammation whereas consumption of refined cereals, red meat, butter, processed meat, high-fat dairy, sweets, desserts, pizza, potatoes, eggs, hydrogenated fats, and soft drinks, are found to be strongly and positively associated with systemic inflammation[46] [72].
Looking at this matter from a dietary level rather than a nutritional level, the Mediterranean diet has a high anti-inflammatory micronutrients and phytochemical content such as n-3 fatty acids, flavonoids, carotenoids, and vitamins C and E [47][73]. Evidence shows that higher adherence to the Mediterranean diet is linked to a lower inflammatory status [48][74]. As a result, applying an intervention to increase the adherence to a Mediterranean diet pattern may have health benefits by reducing systemic inflammation [47][73]. In this regard, also more general, several studies have linked diet quality to inflammation. For example, breast cancer survivors with better postdiagnosis diet quality showed lower CRP levels (1.6 mg/L vs. 2.5 mg/L) and higher scores on the Healthy Eating Index (2005)[49] [75]. Likewise, Orchard et al. observed that a higher HEI-2010 score was strongly associated with reduced IL-6 and TNFR-2 levels in breast cancer survivors [50][76].
Another approach to affect the nervous system’s neuroimmune function is through metabolic alterations[22] [47]. According to mounting evidence, oxidative stress can activate and maintain pain pathways via activating glutamatergic transmission and numerous inflammatory pathways (which are important for the development of peripheral and central sensitization), as well as directly influencing nociceptive centers in the brain [51][77]. Oxidative stress has been proven to be a significant contributor to the pain caused by chemotherapy-induced peripheral neuropathy (CIPN) [52][78]. The findings in mouse models showed that cisplatin-induced mechanical hypersensitivity is caused by peripheral oxidative stress sensitizing mechanical nociceptors, whereas paclitaxel-induced mechanical hypersensitivity is caused by central (spinal) oxidative stress maintaining central sensitization that abnormally produces pain in response to Aβ fiber inputs [52][78]. Furthermore, mitochondrial dysfunction caused by cancer cells (induced by the mitochondrial genome alterations, the associated oxidative stress etc.) [53][79] may play a role in chronic pain. Maintaining mitochondrial function has been proposed as a possible treatment technique for treating or preventing chronic pain[54] [80]. For example, strategies that improve mitochondrial function have shown success in preventing and reversing CIPN in pre-clinical animal models and have begun to show some progress toward translation to the clinic [55][81]. Although dietary intake ultimately directs metabolism, only a few studies showed how metabolic pathways influenced by diet may have a role in the immune activation seen in chronic pain [56][82]. It is asserted that diets high in fruit and vegetable consumption can decrease oxidative stress [46][72]. For example, antioxidants produced from food, such as vitamin A, CoQ10, vitamin E, and vitamin C, have been demonstrated to play an important role in preventing oxidative stress, and several studies have found a link between the consumption of specific foods or food groups and plasma/serum antioxidant capacity[57] [83]. In women who have had breast cancer, it has been demonstrated that drinking fresh carrot juice on a daily basis is a simple and effective way to increase plasma total carotenoids and, as a result, reduce oxidative stress, but not inflammatory markers [58][84].
Additionally, in vitro evidence demonstrated the role of nuclear factor-kappa B (NF-κB), which has a critical role in cancer development and progression [58][85], as well as in regulating inflammatory pain [59][86]. Evidence also found that tomato extracts inhibited TNFα induced NF-κB activity in the androgen-independent human-derived prostate cancer cells [60][87].
Taking into account the links between chronic pain, inflammation, and metabolic dysregulation, and their subsequent impact in cancer survivors, a strategic dietary intervention for this population that could modulate this pathophysiology is worth looking into [22][47]. However, specific evidence for these mechanisms in cancer survivors is yet to be generated and represent an important area for future research.
5. Impact of Diet and Nutrition on Pain in Cancer Survivors through Gut Microbiota
Dietary composition and amount play a significant role in gut microbiota composition and function [61][37]. It has been shown that lesser gut microbial diversity is associated with poorer nutritional status, frailty, comorbidity, and inflammation indicators [62][97]. Based on animal and human studies, dietary intake is seen to be a main short-term and long-term regulator of the gut microbiota structure and function [63][98]. To illustrate; some findings point to a relationship between Vitamin D insufficiency and altered nociception, presumably through molecular processes affecting the endocannabinoid and associated mediator signalling systems [64][99]. Additionally, short-chain fatty acids (SCFAs), which are microbial metabolites, interact with vagal afferents, and impact inflammation and hormonal control may also affect the peripheral immune system to modulate brain function [65][100]. Hence, targeting gut microbiota by dietary intervention is one of the innovative and possibly productive options for chronic pain therapy [66][91].
