Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Timothy Oladiran Ajiboye and Version 2 by Lindsay Dong.
Dithiocarbamate ligands have the ability to form stable complexes with transition metals, and this chelating ability has been utilized in numerous applications. The complexes have also been used to synthesize other useful compounds.
- dithiocarbamate
- metal complexes
- medical use
- industrial applications
- agricultural applications
1. Introduction
Dithiocarbamates are amides formed from dithiocarbamic acid and they have the ability to form stable metal complexes as a result of their exceptional coordination properties [1]. They could generally be classified as heterocyclic dithiocarbamates, symmetric dithiocarbamates, unsymmetric dithiocarbamate, dialkyldithiocarbamates and monoalkyldithiocarbamates [2]. Several methods have been used to synthesize dithiocarbamate compounds. However, the synthesis is commonly achieved by the reaction of carbon disulphide and amine (primary or secondary). The reaction is usually carried out in the presence of electrophiles such as imines, transition metals, epoxides and alkyl halides [3]. The synthesis could be effected without a catalyst or in the presence of an appropriate alkali. Their ligands can form complexes with octahedral, square planar or tetrahedral geometry depending on the type of metal ion and also the ratio of the metal-to-ligand [2]. Dimers of dithiocarbamates are also formed by using dilauroyl peroxide as the oxidizing agent [4] (equation o). Other polyfunctional ligands of dithiocarbamate exist but they are rare compared to other forms of dithiocarbamate compounds [5]. Both the dithiocarbamate ligands and complexes are useful in several applications. However, when both ligands and complexes found relevance in similar applications, the complexes appear to be more potent than the ligands. For instance, dithiocarbamate complexes are more active against microbes than the ligands from which the complexes are formed [6]. The choice of dithiocarbamates compared to other related compounds is attributed to its poor solubility in water, ease of preparation under laboratory conditions, and formation of more stable compounds than several complexes made from other common analytical ligands [7].
2. Heavy Metals Concentration and Remediation
2.1. Heavy Metals’ Removal from the Environment through Dithiocarbamate Compounds
The ability of dithiocarbamate to selectively and strongly bind to most metal ions to form organometallic complex makes them a useful candidate for removing heavy metals from the environment [8][9][14,15]. The presence of two sulphurs with lone pairs of electrons makes it possible for dithiocarbamates to form chelate with these metals as well. However, it is possible for dithiocarbamate to use one of the sulphur donor atoms to form a bond with the metals. In short, it can act as bidentate or monodentate ligand [8][14]. Another factor that makes them particularly useful for metals with a variable oxidation state is their ability to stabilize these metals irrespective of their oxidation states and this can be explained by the oxygen bonding ability of the conjugates formed by dithiocarbamates [10][11][16,17]. The possibility of sharing electrons between the metal ions, sulphur atoms and nitrogen atoms coupled with the formation of metal complexes that cannot dissolve in water also makes them a better heavy metal chelator from the environmental samples [12][18]. The efficiency of heavy metals removal depends on the type of dithiocarbamate used for metal chelating. For instance, the metal chelating ability of diphenyldithiocarbamate ligands was found to be better than the chelating ability of diethyldithiocarbamate that did not contain a phenyl group [12][18]. Apart from the use of dithiocarbamates in the removal of heavy metals, they have also been used to determine and concentrate heavy metals instead of using surfactants [13][14][15][29,30,31].2.2. Trace Elements Concentration and Determination through Dithiocarbamate Compounds
The determination of trace metals usually involves separation and pre-concentration stages. Dithiocarbamate compounds have been used for these purposes and this could be attributed to their selective and chelating properties. Activated carbon coated with phenylpiperazine dithiocarbamate was successfully used to concentrate Pb, Cd, Cu and Mn before they were determined by the flame atomic absorption spectrophotometry (FAAS) method [16][32]. Ammonium pyrrolidine dithiocarbamate, on glass fibre base, was also used to form a chelate complex with metal ions, which was followed by methyl isobutyl ketone elution and atomization of the metal ions. The quantification of the atomized sample was then carried out through high performance liquid chromatography (HPLC) [17][33]. When the multi-element determination of heavy metal ions was carried out through HPLC, dithiocarbamate was included in the column to improve the performance of the method [18][34]. Dithiocarbamate-modified silica gel was also employed for pre-concentration and separation of ions of several precious metals prior to their determination via inductively coupled plasma [19][35].3. Application of Dithiocarbamate Compounds as Stationary Phase in Chromatography
Dithiocarbamate compounds were also used as a component of the stationary phase during ligand exchange chromatography. They were useful for this application due to their strong chelating ability. Yeh and co-workers [20][42] utilized dithiocarbamate coated on silica as the stationary phase in the separation of heavy metals. It was observed that the amount of mercury taken up by this stationary phase was high, which could be attributed to the presence of extra complexing-nitrogen atoms from dithiocarbamate present in the stationary phase. In the chromatographic determination of multiple heavy metals, diethyldithiocarbamate and pyrrolidinedithiocarbamate were deposited on the Sep-Pak cartridge, which was used as the stationary phase. The method was able to determine these heavy metals even at μg l−1 level [21][43].
4. Application of Dithiocarbamate Compounds as Catalysts
4.1. Application of Dithiocarbamate Compounds as Catalyst in Organic Transformation
Core/shell nanostructures have been functionalized with magnetic dithiocarbamate deposited on gold and utilized as the catalysts for synthesizing propargyl amines through A3 coupling reaction [22][44]. The catalyst displayed good performance for the synthesis of propargyl amines when phenylacetylene, benzaldehyde and morpholine were used as the starting material. Further probe into the mechanism of the reaction showed that the reaction proceeded through a process involving the formation of iminium ion intermediate and C-H activation. The choice of metal dithiocarbamate was as a result of its good solubility in organic solvents, chemical stability and the fact that it can be easily used in the anhydrous form [23][24][45,46].4.2. Application of Dithiocarbamate Compounds as RAFT Agent in Polymerization
Simultaneous control of stereoregularity and molecular weight of polymers is beneficial in polymer synthesis but it is difficult to achieve [25][48]. The use of RAFT (reversible addition–fragmentation chain transfer) agents has made simultaneous control feasible and different dithiocarbamate compounds have been investigated as RAFT agent [26][49]. Nitrogen-containing dithiocarbamates are now being used as the most effective RAFT agent with reduced bulky attachment when compared to other RAFT agents [27][50]. The presence of nitrogen in the dithiocarbamate compound stabilizes the cationic intermediate due to the fact that nitrogen is an electron-donating atom [25][27][48,50]. Dithiocarbamate was also used as both emulsifier and RAFT agent in the polymerization of stable latex of vinyl acetate polymer [28][51]. They are often used along with other RAFT agents for better control of tacticity and molecular weight.
5. Application of Dithiocarbamate in Synthesis
5.1. Application of Dithiocarbamate Compounds as Precursors in Material Synthesis
Different synthetic methods have been used to produce metal sulphide nanoparticles and one of these methods involves the use of metal complexes as single source precursors (SSP). Among the metal complexes used as SSP, dithiocarbamate complexes have being the most explored complexes. SIn our laboratory, we have synthesized some dithiocarbamate complexes, which were thermolyzed to generate metal sulphides [29] have been synthesized[52]. The use of dithiocarbamate complexes for the synthesis of these nanoparticles is preferred since dithi ocarbamate is rich in sulphur; hence, the use of a separate sulphur source will not be required [30][54]. Generally, the synthesis from the dithiocarbamate complex using the solvothermal method requires the use of capping agents such as oleylamine, octadecene, dodecane thiol, ethylene glycol and hexadecylamine. Their presence in the system controls the growth of the nanoparticles [31][55], while some of these capping agents (such as oleylamine) can also function as reducing agent, solvent or surfactant in the material synthesis [32][56].5.2. Application of Dithiocarbamate Compounds in the Synthesis of Organic Intermediates
The light-catalyzed reaction of dithiocarbamates in cyclohexane or chlorobenzene solvent leads to the formation of dithiocarbamate-containing lactam. The fact that the product contains dithiocarbamate makes it suitable for other dithiocarbamate-based applications [33][69]. Diethyldithiocarbamate has been used for the synthesis of ferrugine through a reaction that involves refluxing in the presence of cyclohexane and light [34][70].6. Application of Dithiocarbamate Compounds in Agriculture
6.1. Application of Dithiocarbamate Compounds as Herbicides
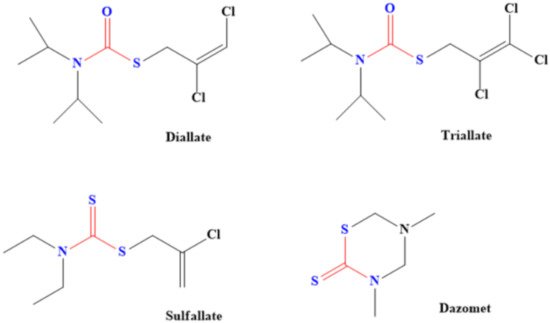
Dithiocarbamate-based herbicides contain groups such as dimethyldithiocarbamate, ethylenebis(dithiocarbamate) and propylenebis(dithiocarbamates). Examples of dithiocarbamate-containing herbicides are Metiram, Dazomet, Thiram, Disulfiram, Propineb, Maneb, Ziram and Zineb [35][77], although some of them are also used as pesticides. These herbicides are majorly used to prevent the growth of some broadleaf weeds as well as plants such as crabgrass, cheatgrass, bromegrass and foxtail [36][78]. Even plant that generates oxidants (active oxygen species) was successfully eliminated through dithiocarbamate herbicides [37][79]. Adjustment of the lipophilic and hydrophilic properties of dithiocarbamate by introducing groups such as sodium salts of dibutyldithiocarbamic acids, hexyl (2-(2- ethoxyethoxy) ethyl) dithiocarbamic acid, butyl (2-(2-ethoxyethoxy) ethyl) dithiocarbamic acid and ethyl (2-(2-ethoxyethoxy) ethyl)-dithiocarbamic acid was found to aid the action of dithiocarbamate as the pesticide. This is because of better penetration of plant cuticles compared to when ordinary sodium diethyldithiocarbamate was used as the herbicide [37][79]. Diallate, Sulfallate, Dazomet and Triallate are other common dithiocarbamate-based herbicides (Figure 16). Diallate [S-(2,3 dichloroallyl-)diisopropylthiocarbamate] is used to control monocotyledon weeds and it acts by attacking their fatty acids [38][80].
Figure 16. Examples of common dithiocarbamate-based herbicides. (One of the sulphur in dithiocarbamate has been replaced in diallate and triallate).
6.2. Application of Dithiocarbamate Compounds as Pesticides
Pesticides made from dithiocarbamates are used as fungicides for various crops during processes such as shipment, storage and growth [39][81]. The structures of some of these dithiocarbamate-based pesticides are shown in Figure 27. These pesticides also kill the larva of some pests that cause plants’ and farm animals’ diseases, thereby boosting food security. For instance, both tricyclohexyltin and triphenyltin N-n-butyldithiocarbamate have been used as larvicide against the larva of Aedes aegypti and Anopheles stephensi mosquitoes [40][82]. These dithiocarbamates were found to be effective against the larva of these mosquito species. Moreover, Meloidogyne incognita, which is a disease caused by nematode, was eradicated by using dithiocarbamate derived from chitin oligosaccharide [41][83]. The derived dithiocarbamate pesticide has high activities for eliminating the nematode. In addition, it inhibits the hatching of eggs, thereby decreasing the population of the nematodes [41][83].
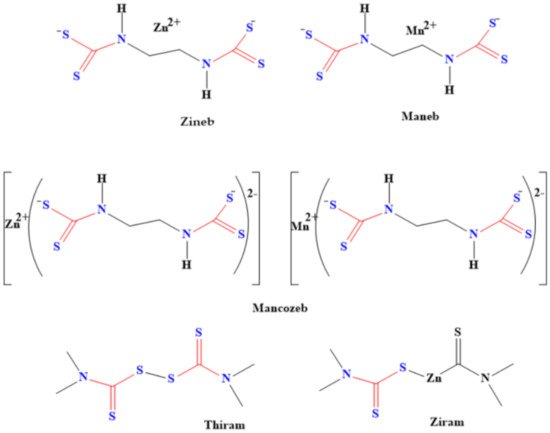

Figure 27.
Examples of dithiocarbamate pesticides.
7. Medical Applications of Dithiocarbamate Compounds
The use of dithiocarbamate compounds in medicine has been investigated for more than 40 years [42][96]. One such application is their use as anti-angiogenic agent and they are usually evaluated for this application by studying their potential to heal wounds. For example, thalidomide dithiocarbamate was evaluated for wound healing to confirm its usage as the anti-angiogenic agent [43][97]. Dithiocarbamate ligands and complexes have also been studied for magnetic resonance imaging and other radiopharmaceutical imaging [42][96]. Gold nanoparticles functionalized with biomimetic amino acid dithiocarbamate were used as nanoprobe for cell imaging as a result of their negligible toxicity to human cells. This dithiocarbamate compound showed an enhancement factor of 9.8 × 105 when used for surface-enhanced Raman scattering imaging [44][98]. Generally, the medical applications could be ascribed to their ability to form metal chelate and the high reactivity of dithiocarbamate anions to other moieties (such as thiol) [44][45][98,99]. Other medical applications of dithiocarbamate, are suwhich are discussed in this review, are summarized in Figure 38.
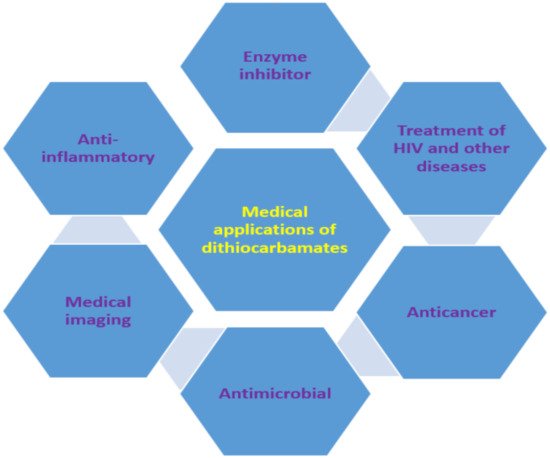

Figure 38.
Medical applications of dithiocarbamate compounds.
8. Application of Dithiocarbamate Compounds in the Industries
Several industries are using dithiocarbamate as the starting materials in different industrial processes and this has spiked the consumption of dithiocarbamate compounds. Some of the industrial uses of dithiocarbamate compounds are shown in Figure 411.
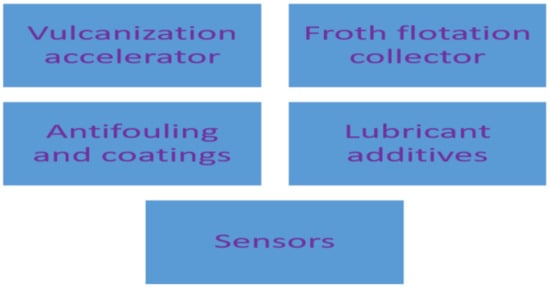

Figure 411.
Industrial applications of dithiocarbamates.
9. Challenges Associated with the Utilization of Dithiocarbamates
Dithiocarbamates that possess aliphatic chains are vulnerable to acid hydrolysis and liberate CS2 under acidic or neutral conditions. In a very strong alkaline condition, the aliphatic dithiocarbamates degrade to give mixtures of sulphur-containing compounds such as sulfonates and disulphides [46][8]. Catalytic oxidation of thiols by dithiocarbamate compounds, leading to the inhibition of pro-apoptotic enzymes, has been reported [47][226]. Dithiocarbamate compounds also play significant roles in the disruption of the developmental stage of aquatic animals [48][227]. The product of metabolic degradation of dithiocarbamate (carbon disulphide) also causes notochord distortions in zebra fish [49][228]. Dithiocarbamate compounds have been found to possess biocidal and cytotoxic properties. Their cytotoxity was discovered to be related to their structures [50][229]. Disulfiram, thiram and mancozeb cause changes in the cell membrane and block glutamate from binding to the receptor, which results in toxic effects on the brain [51][230].
10. Future Perspectives
For future research, the use of dithiocarbamate complexes in medical imaging is still at the infant stage and it needs to be further explored. For instance, the possibility of using dithiocarbamate compounds to solve the problem of scattering, sensitivity and absorption in medical imaging should be investigated. Toxicity of the metal dithiocarbamate complexes should be thoroughly investigated prior to their use for these applications, so that any possible cytotoxic effect that could emanate from the introduction of dithiocarbamate into the ecosystem could be mitigated for the protection of aquatic and terrestrial lives. Moreover, the fate of the unused dithiocarbamate in the environment and their degradation mechanism through the use of photocatalysis and other removal methods should be studied. Furthermore, the effect of dithiocarbamate on the root exudates of common food crops such as maize and soy bean should be investigated so as to enhance food safety and productivity. While iron dithiocarbamate has been investigated for the removal of nitrogen oxides from air samples [52][231], this investigation needs to be carried out on other air pollutants. Finally, renewed efforts should be geared towards the synthesis of novel dithiocarbamate ligands and complexes.
