Carbon monoxide (CO) is a colorless, odorless, and tasteless gas, resulting mainly from the incomplete combustion of fossil fuels and thus largely spread in urban environments or regions with a high traffic density. The sensors that can detect toxic gas are usually characterized by the presence of CO absorption sites in their structures, with the Langmuir reaction model offering a good description of the reaction mechanism involved in capturing the gas.
1. Introduction
The main contributors to the onset of diseases such as stroke, lung cancer, or acute respiratory diseases include carbon monoxide (CO), nitrogen oxides (NOx), sulfur oxides (SOx), and ammonia (NH3). The selective detection and monitoring of air, both from the ambient and indoor locations, could lead to great strides in limiting the exposure of people to toxic quantities of such gases, ensuring a favorable environment for development and growth.
Both the molecular structure and chemical activity of carbon monoxide assure that irreversible bounds are formed when it interacts with hemoglobin, blocking the reaction sites that were originally meant for CO2. The resulting carboxyhemoglobin hinders gas exchange between carbon dioxide and oxygen, which can lead to death by functional asphyxia. Meanwhile, large concentrations, 0.1% for 1 h, of CO are lethal; the more problematic aspects of CO are its effects on the human health and psyche when under continuous exposure at low concentrations. Such cases routinely affect the central nervous system, reducing visual and physical capacity, lowering coordination, and introducing lapses in concentration. Thus, the World Health Organization (WHO) has prescribed exposures to CO of no more than 8 h at 9 ppm and 1 h at 26 ppm [1].
According to the IUPAC, chemical sensors can be classified according to their transduction mechanism [2] into three main classes: (i) systems that detect changes in the electrical and electrochemical properties of an analyte, (ii) systems that monitor changes in their physical properties, and (iii) sensors that measure the optical absorption of chemical analytes [3].
Prior research has identified composite materials, a solution of metal oxides, carbon nanostructures, and conducting polymers, as being effective films in the detection of toxic gases and in particular CO. The detection of gases by chemoresistive sensors has received great attention because of their advantages over the other sensors (electrochemical, optical): low cost, long lifetime, high sensitivity, fast response time, and small sizes [4].
Carbon nanomaterials, such as single-walled carbon nanotubes (SWCNTs), pristine carbon nanomaterials, multi-walled carbon nanotubes (MWCNTs), and graphene, have been extensively used as the active layers for the development of chemoresistive sensors [5][6][7][5,6,7].
2. Chemosensors: An Overview on CO Detection
A versatile subset of chemosensors, those based on a composite material sensing layer, are primarily composed of three elements: metal oxides for the selective reaction with a target gas, carbon nanostructures for increasing the working area, and
conductive polymers (CP
) for increased stability and easier delivery of the signal to the transducer. Metal oxides refer to those p or n-type semiconductor materials, such as ZnO, SnO
2, or CuO, that have good chemical stability, high electron mobility and that allow for easy control of their morphological properties. Their primary function consists in the absorption and reduction of a gas on their surface, which is followed by the measurement of any resulting change in the electrical conductivity and resistance of the sensing layer. While such sensing layers are highly sensitive, large-scale implementation has been previously limited by their high operating temperatures (300–500 °C)
[8][9][10][14,15,16].
NCMs consisting of combinations of metal oxides with carbonaceous materials such as reduced graphene (rGO), MWCNT, SWCNT, or other CNTs-based structures were the primary solution to the concerns regarding high temperature operation. Such structures operate at room temperature without a loss in sensitivity, thus proving a superior alternative to sensors solely based on metal oxides. Further enhancements in performance have been observed on doping a CP using metal oxides while at the same time functionalizing the polymer by use of carbon nanostructures. The use of functionalized conducting polymers such as polyaniline (PANI)PANI, polythiophene (PTh), polypyrrole (PPy)PPy, and poly (3,4-ethylenedioxythiophene) (PEDOT)PEDOT leads to larger sensing surface areas and an increase in conductivity of the sensing layer due to the presence of many suspended bands and defects at their surface. However, such sensors are sensitive to changes in humidity, with high operating temperatures being employed to reduce its interference for simple metal oxide sensors and chemical alterations of the sensing surface being employed for composite materials.
The use of conductive organic polymers has shown increased performance in the detection of gases, being highly synergistic with the functionalizing carbon nanostructures and preserving the individual properties of the constituting components. Polymers such as PANI, PTh, PEDOT, and PPy have shown superior electrical and mechanical properties when combined with CNTs or rGO, improving sensitivity by up to 100%
[11][12][13][17,18,19]. However, to achieve those high-performance parameters, either a good adhesion of the metals to the CP or a high homogeneity of the CP solution needs to be assured.
3. Sensing Principle
3.1. PANI Structure and Conductivity
PANI is an intrinsically conductive polymer that owes its electrical conductivity to the presence of a π-type electronic conjugation in its structure. One of the most studied polymers in the last 20 years, it can be employed for the detection of CO and other gas molecules such as NH
3, H
2S, and H
2 having been integrated in both electronic and optical sensors
[14][15][16][22,23,24].
The conductive structures of PANI are predominantly composed of imine groups, although amine groups can also appear when the polymer is protonated in the presence of an acid or a dopant. This leads to the formation of polarons and bipolarons that are responsible for preserving the conductivity of PANI on substrates.
3.2. PANI Sensing Mechanism on CO Exposure
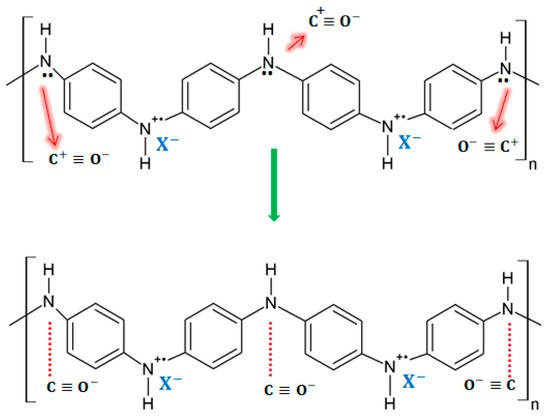
On exposure to CO, the response of a PANI-coated sensor consists of a decrease in the electrical resistance of the sensing layer due to the partial charge transfer between the amino nitrogen (–NH) structure in the polymer and the carbocation present in CO. Then, the transferred charge extends along the polymer chain, thus leading to an increase in the conductivity of the layer. A diagram of the sensing mechanism for the exposure of PANI to CO can be seen in
Figure 13.
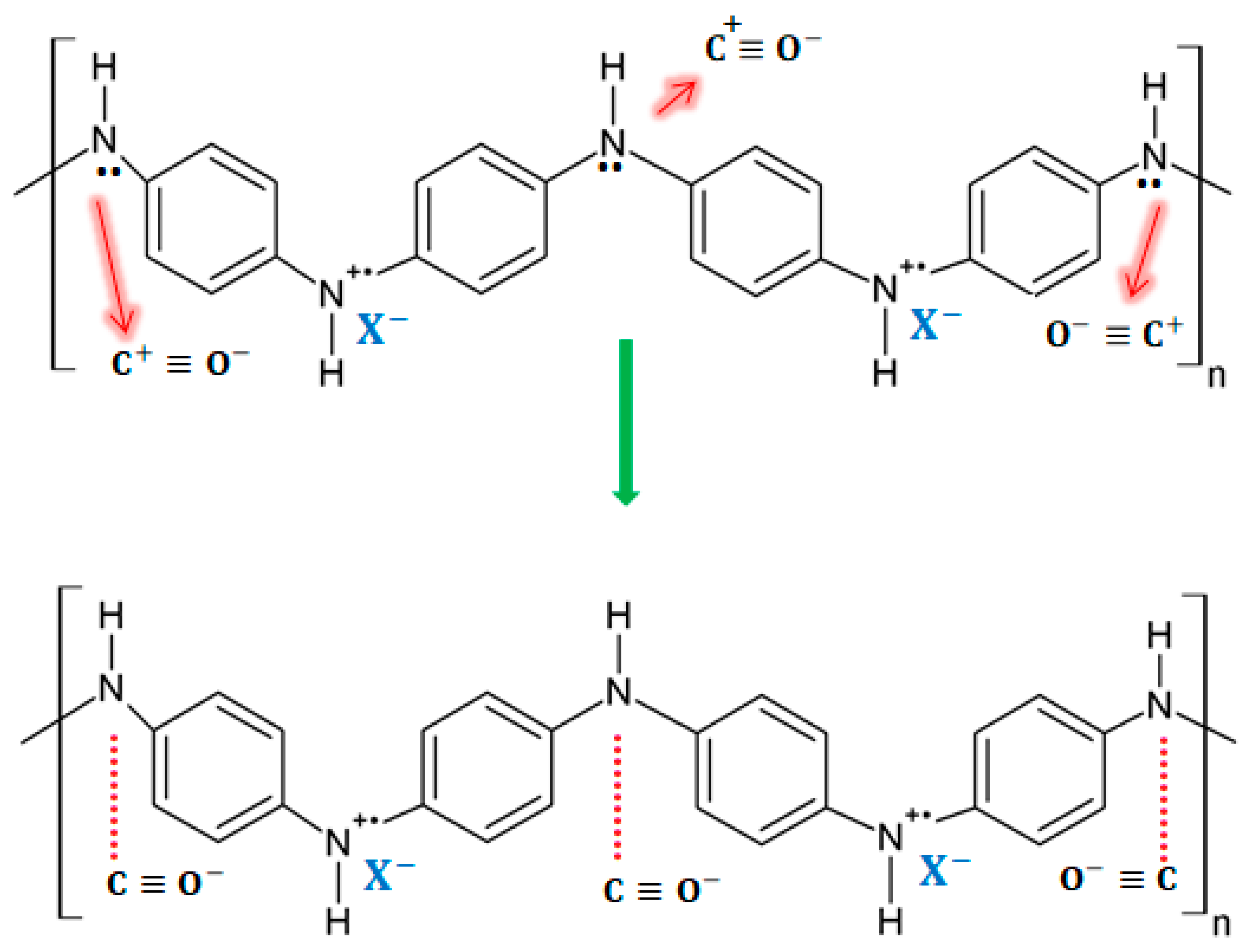
Figure 13. Sensing mechanisms for PANI in the presence of CO [17]. Sensing mechanisms for PANI in the presence of CO [31].
While most chemosensors based on PANI layers quantify the concentration of a target gas based on resistive measurements, forays have been made into other metrics such as current flow on CO exposure
[15][16][23,24].
3.3. PANI–NCM Structure Sensing Mechanism on CO Exposure
Current technologies functionalizing CPs with NCMs are restricted to the resistive measurements of changes in a target gas’s concentration. For polymeric structures that include insertions of CNTs, a drop in resistance is detected upon exposure to CO.
3.4. PPy Structure and Conductivity
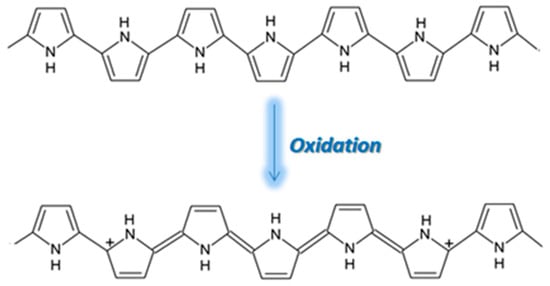
PPy is an attractive alternative to PANI as a CP for gas sensing, displaying excellent sensitivity to gas molecules, tunable conductivity, a low production cost, and the ability to function at room temperature
[18][37]. However, the use of PPy is not widespread due to its poor stability, the oxidation of PPy being highlighted in
Figure 27. During oxidation, the removal of π electrons in the conjugated bond leads to a local relaxation of the benzenoid structure into a quinoid structure, thus creating a pair of radicals. This, together with the appearance of a positive charge, leads to the formation of bipolarons, leaving only two cations in the PPy ring. Those cations can move through the π electronic cloud, thus generating the conduction of electron through the sensing layers. Thus, unlike PEDOT and PANI, when PPy reacts with gases that possess electron acceptors, the electrons from its aromatic ring are removed and the conduction is enhanced
[19][20][21][38,39,40].
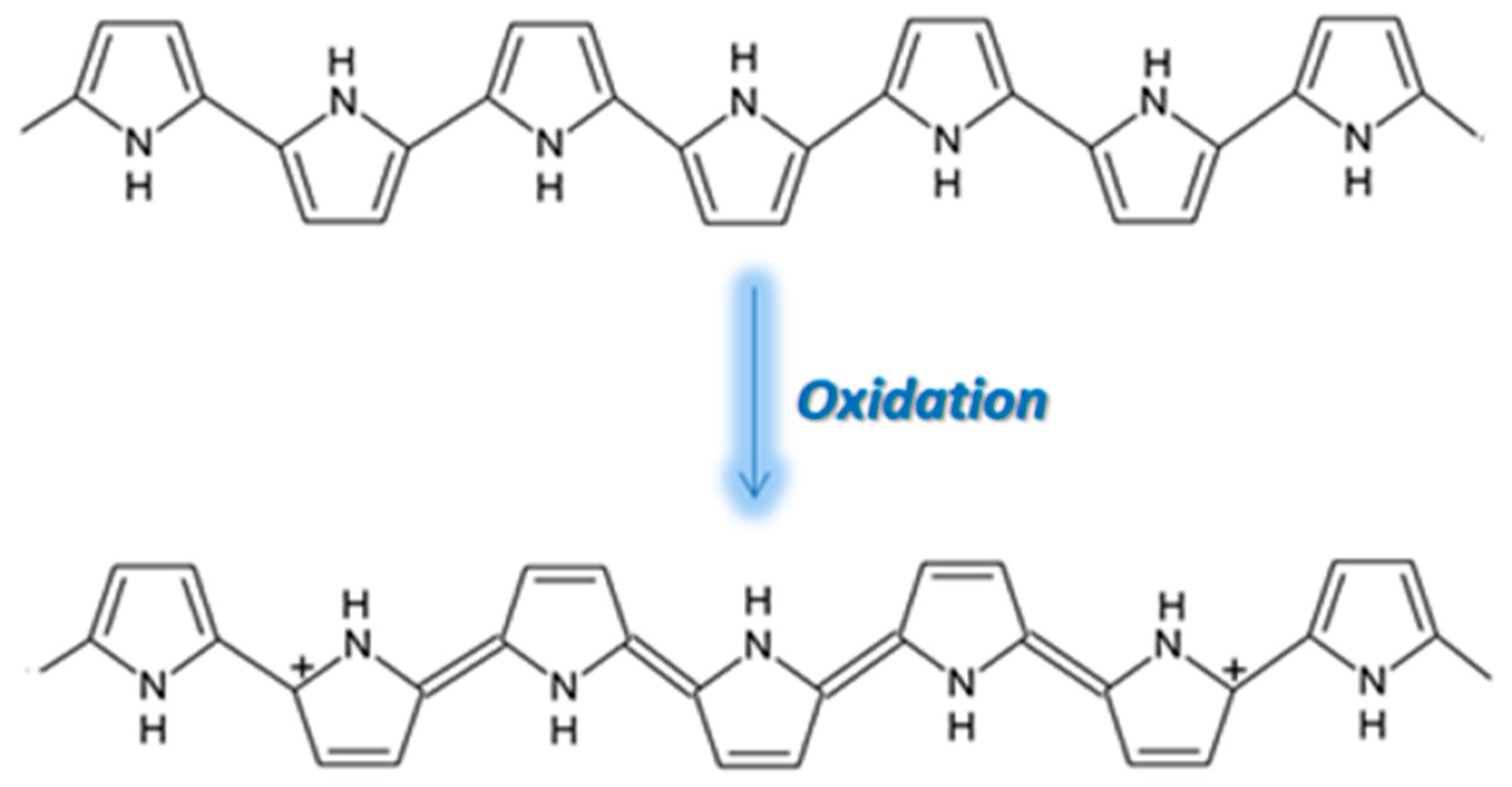
Figure 27. The oxidation of PPy [22]. The oxidation of PPy [41].
4. Formulation Strategies
The use of CPs, with and without carbon materials, has two disadvantages: poor solubility in common solvents and mechanical instability, especially for PANI, due to changes during the oxidation and reduction reactions occurring during the doping process. One approach to improving solubility is to combine CPs with various hydrophilic polymers (or polyelectrolytes) such as
polystyrene sulfonate (PSS
), polyacrylic acid (PAA), polyvinylpyrrolidone (PVP), PAA, PVP, and
polyethylene glycol (PEG)PEG, resulting in composite solutions such as PANI:PSS, PANI:PEG, PANI:PVP, PEDOT:PSS, and PPy:PSS
[23][24][25][26][27][28][59,60,61,62,63,64].
Phongphut et al. presented the role of PSS, a co-solvent and dopant polyelectrolyte, in making a nanocomposite inkjet solution with PEDOT:PSS on carbon electrodes, with the resulting compound showing improved solubility of the polymer in aqueous media
[29][65]. These electrolytes were also used for NCMs dispersions.
5. Conclusions and Future Perspectives
An analysis on the performances and sensing mechanisms of CO detectors based on CPs and carbon composites has been conducted. Most of the sensors
analyzed in the review showed CO absorption sites in their structure, with the Langmuire adsorption model offering a good description of their reaction mechanism. While sensors using NCMs based on metal oxides showed a non-uniformity in their resistive change to the presence of CO, with either an increase or decrease being possible, CP-based sensing layers have been found to consistently be characterized by a decrease in resistance. This consistency is due to the physical expansion of the polymer layer as well as due to the enhancement of the electron transfer properties resulting from the functionalization with CNTs. Thus, the advantages of using CPs have been determined to include higher selectivity, shorter response and recovery times, and operation at room temperature. However, research still needs to be conducted on the interferences of other gases and the effect of humidity. Based on the presented data, we can conclude that hybrid materials formed by conductive organic polymers combined with carbon nanostructures are desirable materials for use in gas detection, especially in CO detection. Although chemoresistive sensors have been extensively studied in recent decades, there are some aspects that can be improved: methods of preparation and deposition of the sensitive film, both in terms of cost and technology, sensitivity to non-intrinsic gas species, selectivity to a chosen gas, and the degree of miniaturization of sensors and their control devices.