Major advances in pediatric cardiology in recent decades, especially surgical techniques, have resulted in an increasing number of patients with congenital heart disease (CHD) surviving to adulthood. This has generated new challenges, particularly with regards to the late onset of complex arrhythmias. Abnormal anatomy, surgical scarring, chronic hypoxemia, hemodynamic compromise, neuro-hormonal abnormalities, and genetic factors can all contribute to creating a unique substrate for arrhythmia development.
Major advances in pediatric cardiology in recent decades, especially surgical techniques, have resulted in an increasing number of patients with congenital heart disease (CHD) surviving to adulthood. This has generated new challenges, particularly with regards to the late onset of complex arrhythmias. Abnormal anatomy, surgical scarring, chronic hypoxemia, hemodynamic compromise, neuro-hormonal abnormalities, and genetic factors can all contribute to creating a unique substrate for arrhythmia development. This review attempts to synthesize the current state of knowledge spanning the spectrum from underlying mechanisms of arrhythmias in patients with congenital heart disease to current ablative strategies. We discuss existing knowledge gaps and highlight important areas for future research.
1. Introduction
As a result of major advances achieved in the past decades in pediatric cardiology and surgical techniques regarding anatomical correction of congenital defects, most patients with congenital heart disease (CHD) now reach adulthood, such that the population of survivors is increasing and aging
[1]. This success is, however, tempered by the onset of late complications, including arrhythmias that are a major source of morbidity. Projections indicate that 50% of 20-year-old subjects will experience an atrial tachyarrhythmia during their lifespan
[2]. Abnormal anatomy, post-surgical scarring, and systemic factors contribute to establishing a unique substrate for arrhythmia development. The specific substrates, triggers, and modulators for arrhythmias in CHD (
Table 1), discuss current management strategies, including catheter ablation, and offer future perspectives (
Table 2).
Table 1. Arrhythmias pathophysiology in congenital heart disease.
| Substrate and Trigger |
Pathophysiology |
Comments |
| CHD-related substrate |
|
AV, atrioventricular; AVSD, atrioventricular septal defect; ccTGA; congenitally corrected transposition of the great arteries; CHD, congenital heart disease.
Table 2. Management of arrhythmias in congenital heart disease.
| Item |
Management |
Comments |
| -
-
accessory pathways
-
twin AV nodes
|
| diagnosis |
-
ECG
-
Holter-ECG
-
event recorder
-
connected devices (smart watch, handheld ECG)
-
implantable loop recorder
-
electrophysiological study
|
-
displaced conduction pathways mainly in ccTGA, AVSD, univentricular hearts and heterotaxy syndromes
-
Ebstein anomaly associated with a high prevalence of accessory pathways (often multiple)
-
twin AV nodes mainly reported in AV discordance, AVSD and right or left isomerism
|
|
| |
|
post-operative substrate |
-
incisional flutter around right lateral atriotomy is the second most common mechanism (behind the peritricuspid circuit)∙ incisional flutter around right lateral atriotomy is the second most common mechanism (behind the peritricuspid circuit)
-
wide variety of circuits according to the underlying phenotype and previous surgeries
-
ventricutolomy incisions, patches and conduits can favor the occurrence of ventricular arrhythmias
-
four main anatomical isthmuses described in tetralogy of Fallot
|
|
| medical therapy |
-
rhythm control strategy preferred
-
modest efficacy
-
data primarily extrapolated from acquired cardiomyopathies
-
class I drugs generally discouraged
-
amiodarone associated with a high burden of long-term side effects
|
-
antiarrhythmic agents should consider coexisting sinus node or AV node disease, heart failure, associated therapies, child-bearing potential, and comorbidities
|
cardiovascular risk-related substrate |
|
| catheter ablation |
-
advances in technologies and growing experience
-
importance of high-density mapping and image integration
-
significant improvement of outcomes
-
should be considered as first-line therapy
-
long-term recurrences remain common -
interest of targeting all inducible arrhythmias
-
global experience with catheter ablation of ventricular arrhythmia in CHD other than tetralogy of Fallot remains scarce
-
underlying mechanisms and substrates to target in atrial fibrillation are key research priorities
|
|
|
|
-
catheter ablation must be performed in expert centers with multidisciplinary specialized teams
-
remote magnetic navigation of particular interest in complex anatomies
-
transbaffle or transtube punctures used with high-success rate and low rate of complications when performed by experienced operators
|
genetic substrate |
-
important role of genetics in the pathogenesis of CHD
-
genetic abnormalities associated with an increased risk of arrhythmias
-
Several genes identified (e.g., NKX 2.5)
|
|
| perioperative assessment |
-
to treat arrhythmia substrate that will become inaccessible after surgery or to guide surgical ablation
-
in Ebstein patients before surgery (at least in patients with ventricular pre-excitation and systematically in some teams)
-
in Fallot patients before pulmonary valve replacement, electrophysiology study +/− catheter ablation now recommended in patients with history of sustained VT
|
|
triggers for arrhythmias |
|
-
the importance of remodeling in CHD mays be associated with increased abnormal automaticity and/or afterdepolarizations
-
rule-out hemodynamic conditions (regurgitant or obstructive lesions) in patients with new-onset or worsening arrhythmias
-
coronary artery abnormalities or acquired coronary artery disease
-
neurohormonal activation reported in different forms of CHD
-
C-reactive protein associated with arrhythmic events in CHD
|
AV, atrioventricular; CHD, congenital heart disease; ECG, electrocardiogram; VT, ventricular tachycardia.
2. Arrhythmias Pathophysiology and Genesis in Congenital Heart Disease
2.1. Substrates for Arrhythmias in Congenital Heart Disease
2.1.1. Congenital Heart Disease-Related Substrate
Although in most cases arrhythmias in CHD are an acquired condition resulting from surgical scars and other chronic contributing factors, cardiac arrhythmias may also be related to the structural malformation itself. The development of conduction pathways may be impacted by the embryological abnormalities responsible for CHD, and the atrioventricular (AV) node and the His bundle may be displaced beyond the confines of Koch’s triangle. Atypical AV nodal reentrant tachycardia (AVNRT) has for example been reported in patients with complex CHD, in particular cc-TGA and univentricular hearts, with displaced AV nodes and slow pathways
[3][4][3,4]. The prevalence of AVNRT across the various forms of CHD remains, however, poorly characterized.
In some patients, the structural cardiac malformation can be accompanied by accessory or duplicate AV connections with the potential for reentrant tachyarrhythmias
[5]. The most glaring example is the Ebstein anomaly, where accessory pathways have been reported in 10 to 38% of patients
[6]. When present, multiple accessory pathways along the abnormal tricuspid annulus are found in up to 50% of patients, sometimes with complex insertion patterns. Accessory pathways are also prevalent in patients with Ebsteinoid malformations of the tricuspid valve in the setting of congenitally corrected transposition of the great arteries (cc-TGA). Additional types of CHD associated with an increased prevalence of accessory pathway include heterotaxy syndromes, AV septal defects, and some forms of univentricular hearts.
Duplicate AV connections were first described by Mönckeberg in 1913, with two separate coexisting AV nodes, usually called “twin AV nodes”. In the presence of a sling of tissue connecting the two AV conduction systems, so-called Mönckeberg sling
[7], a macroreentrant circuit can arise with a reciprocating tachycardia that courses antegrade by one AV nodal pathway and retrograde via the second AV nodal pathway
[8]. This scenario occurs most commonly in patients with a constellation of congenital heart defects, i.e., AV discordance, malaligned complete AV septal defect, and right or left atrial isomerism.
2.1.2. Post-Operative Substrate
Reentrant tachycardias are frequently encountered after surgical repair of a wide variety of types of CHD. The initiation and maintenance of a reentrant arrhythmia require the presence of myocardial tissue with adjacent tissue having altered electrophysiological properties. Suture lines, patches or prosthetic material provide a core of inexcitable tissue that creates a central area of block, with the potential for reentrant circuits to form around these obstacles. Moreover, delayed conduction across areas of abnormal myocardial tissue allows for the circulating wave fronts to reach adjacent substrates that are no longer refractory, thereby permitting tachycardia circuits to be sustained.
At the atrial level, circuits encountered vary according to the anatomic defect and type of surgical repair. On the whole, cavo-tricuspid isthmus-dependent circuits remain the most common in patients with CHD
[9]. Incisional intra-atrial reentrant tachycardia (IART), in particular around a right lateral atriotomy, is the second most common circuit. In other forms of CHD, such as in patients with older-style Fontan surgery (i.e., right atrium to pulmonary artery connections), long-term hemodynamic stress results in markedly abnormal atrial myocardium prone to various IART circuits around scar areas scattered in the atria. A substantial proportion of arrhythmias encountered have a focal activation pattern and are thought to be micro-reentrant circuits by virtue of their mode of induction and termination and response to pacing maneuvers. The term non-automatic focal atrial tachycardia (NAFAT) is commonly used in this setting to distinguish these arrhythmias from the more standard focal arrhythmias that are due to abnormal automaticity.
Similarly, the propensity to develop ventricular arrhythmias is influenced by the presence of a ventriculotomy and/or a patch or conduit inserted in the ventricle. The most studied example is the tetralogy of Fallot, where four potential anatomical isthmuses that could sustain macroreentrant ventricular tachycardia circuits are well characterized: isthmus 1, bordered by the tricuspid annulus and a right ventricular incision; isthmus 2, between the right ventricular incision and pulmonary valve; isthmus 3, between the pulmonary valve and ventricular septal defect patch; and isthmus 4, between the ventricular septal defect patch and tricuspid annulus. Isthmus 3 is the narrowest and appears to be most commonly implicated substrate in ventricular tachycardia circuits
[10]. Progress in surgical techniques from a classical transventricular to a transatrial-transpulmonary approach may eliminate or alter the geometry of isthmuses 1 and 2 but does not alter the presence of isthmuses 3 or 4.
Lastly, and more anecdotally, accessory AV pathways may be created by surgical intervention, as reported in some patients with tricuspid atresia after a Fontan–Björk procedure connecting the right atrial appendage to the right ventricular outflow tract
[11].
2.1.3. Cardiovascular Risk-Related Substrate
As the CHD population ages, it also appears that factors associated with atrial arrhythmias (mainly atrial fibrillation) in the general population, such as ageing, obesity, hypertension, obstructive sleep apnea, and male gender, are likewise associated with arrhythmias in patients with CHD. In a multicenter study of patients with heterogeneous forms of CHD and atrial arrhythmias, factors independently associated with atrial fibrillation were older age, number of cardiac surgeries, and traditional cardiovascular risk factors
[12]. These factors contribute to electrical and structural atrial remodeling that promotes the genesis of atrial arrhythmias. The importance of considering associated conditions in patients with CHD is increasingly recognized, but while screening for and aggressively managing cardiovascular risk factors and coexisting comorbidities appears to impact favorably outcomes in non-CHD populations
[13], comparable data specific to adults with CHD are lacking.
2.1.4. Genetic Substrate
Appreciation of the role of genetics in the pathogenesis of CHD has increased at a rapid pace over the past 15 years. Epidemiological studies have suggested that a genetic cause can be identified in more than 20% of cases. Single-gene disorders are found in 3% to 5%, gross chromosomal anomalies/aneuploidy in 8% to 10%, and pathogenic copy number variants in 3% to 25% of those with CHD as part of a syndrome and in 3% to 10% among those with isolated CHD
[14]. Genotype/phenotype correlations have revealed that genetic abnormalities could be associated with an increased risk of cardiac arrhythmias, and an interesting example is provided by mutations in the NKX2-5 gene that encodes a homeobox transcription factor known to be involved in a diverse set of congenital heart malformations. The most common clinical presentation associates atrial septal defect with AV block, with a high incidence of sudden cardiac death. Although the predominant cause of sudden death is thought to be related to conduction disorders, an increased risk of tachyarrhythmia is also reported
[15]. Analyses of embryos showed a down-regulation and the abnormal expression of different gap junction proteins (GJas) as an underlying explanation for abnormalities in impulse propagation, and subsequently the development of arrhythmias. Reduced levels of GJa1 were shown to contribute to an increased risk of ventricular arrhythmias and sudden death, while reduced GJa5 levels were linked to atrial electrical instability with increased risk of atrial fibrillation
[16]. Furthermore, a lateral distribution of gap junctions at myocyte junction borders is thought to affect dissipation of the cardiac impulse within the ventricular sink, prolonging its propagation and heightening the risk of arrhythmogenesis through micro-reentry circuits.
2.2. Triggers for Arrhythmias in Congenital Heart Diseases
2.2.1. Abnormal Automaticity and Triggered Activity
Automaticity is the property of cardiac cells to generate spontaneous action potentials. It results from diastolic depolarization caused by a net inward current during phase 4 of the action potential. Under normal conditions, atrial and ventricular myocardial cells do not display spontaneous diastolic depolarization or automaticity. Afterdepolarizations are depolarizations that attend or follow the cardiac action potential and depend on preceding transmembrane activity for their manifestation. Early afterdepolarizations (EAD) interrupt or delay repolarization during phase 2 and/or phase 3 of the cardiac action potential, whereas delayed afterdepolarizations (DAD) occur after full repolarization
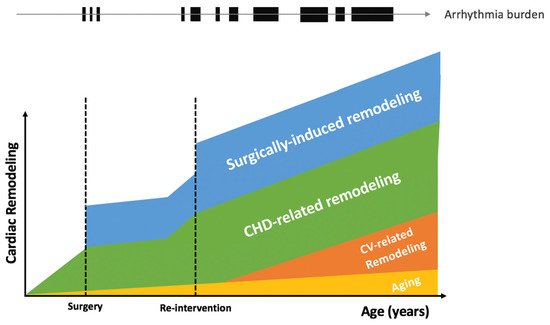
[17]. While triggered activity and abnormal automaticity has not been specifically described in patients with CHD, the importance of progressive structural cardiac remodeling in CHD (
Figure 1) is associated with electrical remodeling involving alteration of ion channels, pumps, and exchangers, that may be associated with increased abnormal automaticity and/or afterdepolarizations
[18]. While focal arrhythmias in CHD are often related to presumed micro reentry mechanisms (NAFAT)
[19], triggered events may also cause automatic tachycardias and arrhythmias with focal activation pattern account for 5–10% of all regular atrial arrhythmias
[9][20][9,20]. Abnormal impulses also give rise to premature beats, which can precipitate tachyarrhythmias by initiating reentrant circuits.
Figure 1. Cardiac remodeling and arrhythmia burden in congenital heart diseases. CHD, congenital heart disease; CV, cardiovascular.
2.2.2. Hemodynamic Alterations
The hemodynamic abnormalities associated with CHD contribute importantly to ventricular and atrial structural remodeling. Left ventricular outflow tract obstruction is a classic example of the role of hemodynamic changes on structural remodeling, with resultant left ventricular overload and increased fibrotic process
[21]. In patients with ventricular septal defects, the left ventricular hypertrophy and extent of fibrosis are independently associated with sudden death
[22]. Years of palliation before surgical repair, non-pulsatile subpulmonary perfusion, single or systemic right ventricles, obstruction to conduits or leaks, and valvular regurgitation are other specific elements that may contribute to hemodynamic derangements in patients with CHD. From a chronological point of view, rather than ageing itself, the longer the time interval prior to surgical repair, the higher the long-term arrhythmia risk. Histology and immunohistochemistry analyses of myocardial tissue resected from 65 CHD patients who underwent cardiac surgery revealed that extent of fibrosis, myocyte diameter, capillary distance, and CD45-positive cell infiltration were correlated with overload duration, and that this time-course related remodeling was greater in patients with a history of arrhythmias
[23].
Hemodynamic and arrhythmic complications are so intimately linked that it is strongly recommended for adults with CHD and new-onset or worsening arrhythmias to rule-out potential contributory conditions such as regurgitant or obstructive lesions. Occasionally, the work-up reveals conditions that should be addressed by transcatheter or surgical interventions. For example, in patients with univentricular physiology, the extracardiac conduit is associated with a lower incidence of atrial arrhythmias, which is thought to reflect, in part, reduced exposure of the right atrium to elevated systemic pressures. Fontan conversion from an atriopulmonary connection to a total cavopulmonary connection combined with arrhythmia operation in patients with symptomatic and uncontrollable arrhythmia episodes is associated with significant functional improvement
[24].
2.2.3. Myocardial Ischemia
Ischemia is a well-known trigger for cardiac arrhythmias: more than 5% of patients with ST segment elevation myocardial infarction develop ventricular fibrillation
[25]. The risk of arrhythmias induced by ischemia varies according to the type of cardiac defect. Coronary artery abnormalities, such as anomalous connection of a coronary artery or coronary artery fistula, are more frequent in patients with CHD. Cases of sudden death have been reported in patients with a left coronary artery that courses between the aorta and the pulmonary artery. Deaths predominantly occur during or just after vigorous exercise, likely due to ischemia resulting from extrinsic coronary artery compression. Other rare coronary abnormalities include the presence of a single coronary artery, coronary atresia, congenital stenosis or atresia of a coronary ostium, and coronary arteries arising from the pulmonary artery. Long-term coronary complications are rare but can also occur in patients with coronary artery reimplantation, such as patients with D-TGA after arterial switch operation following growth and development
[26]. In patients with D-TGA and atrial switch surgery, myocardial perfusion defects have also been described
[27]. The branching pattern of the major coronary vessels is often abnormal, and hypertrophy of the right ventricle develops over years of exposure to systemic pressures. This inevitably increases myocardial oxygen demand that may exceed supply from a single right coronary artery, particularly during rapid heart rates
[28][29][28,29].
Paralleling the rising prevalence of traditional cardiovascular risk factors in the aging population of patients with CHD is a higher burden of coronary artery disease
[30]. The incidence of myocardial infarction has been reported to be greater than in the general population, and associated with higher mortality
[31]. Atherosclerosis may contribute to arrhythmia vulnerability in patients with CHD, and its optimal screening and management should be integrated into a global approach to the CHD patient that considers all potential co-existing conditions.
2.3. Modulators for Arrhythmias in Congenital Heart Diseases
2.3.1. Neurohormonal Perturbations
The autonomic nervous system plays an important role in the modulation of arrhythmogenesis. Sympathetic influences on cardiac electrophysiology are complex. Although sympathetic stimulation has similar effects on both atrial and ventricular myocytes, vagal stimulation does not. In the ventricles, vagal stimulation prolongs the action potential duration and effective refractory period, whereas in the atria, vagal activation reduces the atrial effective refractory period, augments spatial electrophysiological heterogeneity, and promotes EADs toward the end of phase 3 of the action potential. This differential effect may explain why parasympathetic stimulation is proarrhythmic in the atria but antiarrhythmic in the ventricles, whereas sympathetic stimulation seems to be proarrhythmic for both chambers
[32]. Neurohormonal activation has been reported in different forms of CHD, with increased circulating concentrations of atrial natriuretic peptide, brain natriuretic peptide, endothelin-1 renin, aldosterone, norepinephrine, and epinephrine
[33]. Even though asymptomatic subjects had evidence of significant neurohormonal activation, a stepwise increase of these chemical messengers was observed according to functional status and other clinical indices. Interestingly, the level of neurohormonal activation also correlated with electrocardiographic markers, such as QRS duration and QT interval. However, it remains to be demonstrated whether neurohormonal activation has prognostic implications in adults with CHD and whether pharmacological manipulation of neurohormonal systems translates into a clinical benefit on arrhythmia burden.
2.3.2. Chronic Inflammation
Chronic low-level inflammation has increasingly been implicated in cardiovascular disease. Recent evidence demonstrates that medications targeting the inflammatory response can prevent cardiovascular events
[34]. The level of high-sensitivity C-reactive protein has been strongly associated with outcomes in adult CHD patients. In a prospective cohort of 707 outpatients, those with the highest quartile of high-sensitivity C-reactive protein more often experienced the combined outcome of all-cause mortality or non-elective cardiovascular hospitalization (30.5% vs. 11.3%, HR = 2.00, 95% CI 1.35–2.97), all-cause mortality (11.9% vs. 1.5%, HR = 4.23, 95% CI 1.87–9.59), and arrhythmic events (HR ~ 2) during an average follow-up of 815 days
[35]. These findings implicate inflammation in the pathophysiology of arrhythmia development among adults with CHD. While the underlying mechanisms involved require further study, a better understanding of the causes of inflammation and pathways by which inflammation results in adverse outcomes could potentially identify promising pharmacologic targets.