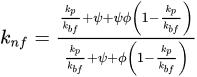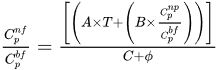Energy consumption in the industrial sector can be significantly reduced by improving heat transfer rates in heat exchanger circuits, pool boiling, metal cutting industries, etc. Numerous energy-related issues can be overcome to a large extent by improving heat flow properties by utilizing nanofluids. As to the improvement in thermophysical properties of metal oxide-based nanofluids, key parameters affecting the thermophysical properties of nanofluids, such as particle volume fraction, temperature, particle size and various stabilizers, were involved. The importance of DLVO theory and zeta potential to control the electrostatic repulsion and pH values of nanofluids for stable nanofluid formulations were highlighted. It has been observed that classical theories of thermal conductivity and viscosity cannot predict exact values for a wide range of variables. Therefore, various extensive correlations have been introduced to predict the thermophysical properties of nanofluids.
- thermal conductivity
- zetapotential
- viscosity
- specific heat
- wettability
- pool boiling
- energy efficiency
1. Introduction
2. Effect of Metal-Oxide Based Nanofluids on Thermophysical Properties
2.1. Specific Heat
2.1. Specific Heat
Specific heat capacity (SHC) of a nanofluid is defined as the capability of the nanofluid to absorb heat energy without any phase change. It quantifies various essential thermal behaviors such as the rate of heat transfer, the efficiency of the heat exchanger and the average Nusselt number. Specific heat capacity is critical to the accurate design and assembly of heat transfer applications through cooling and lubrication systems, such as solar collectors, refrigeration and air conditioning, machining, etc.| Maxwell | |
| [ | 13] |
| Burggeman | |
| [ | 3325] |
| Timoofeva | |
| [ | 3429] |
| Sujith et al. | |
| [ | 3527] |
|
|
You and Choi |
| [ | 3628] |
| Hamilton-crosser | |
| [ | 3724] |
|
|
Koo and Kleinstreuer |
| [ | 3830] |
| Sadik et al. | |
| [ | 3931] |
| Liu and Lin | |
| [ | 4032] |
| Models/Correlations | Author |
|---|
2.3. Viscosity
2.3. Viscosity
The study of a fluid’s response to the applied shear stress is called rheology [54]. Shear stress versus shear rate gives the rheological behavior of a particular fluid. The ratio of shear stress and strain rate is termed as viscosity, and this determines the rheological properties of a fluid. In general, fluids are categorized into Newtonian and non-Newtonian types. Newtonian fluids exhibit a linear relation between the shear rate and shear stress. While non-Newtonian fluids show a nonlinear relationship, and the fluid flow curve does not intersect the center of the axes of the coordinate system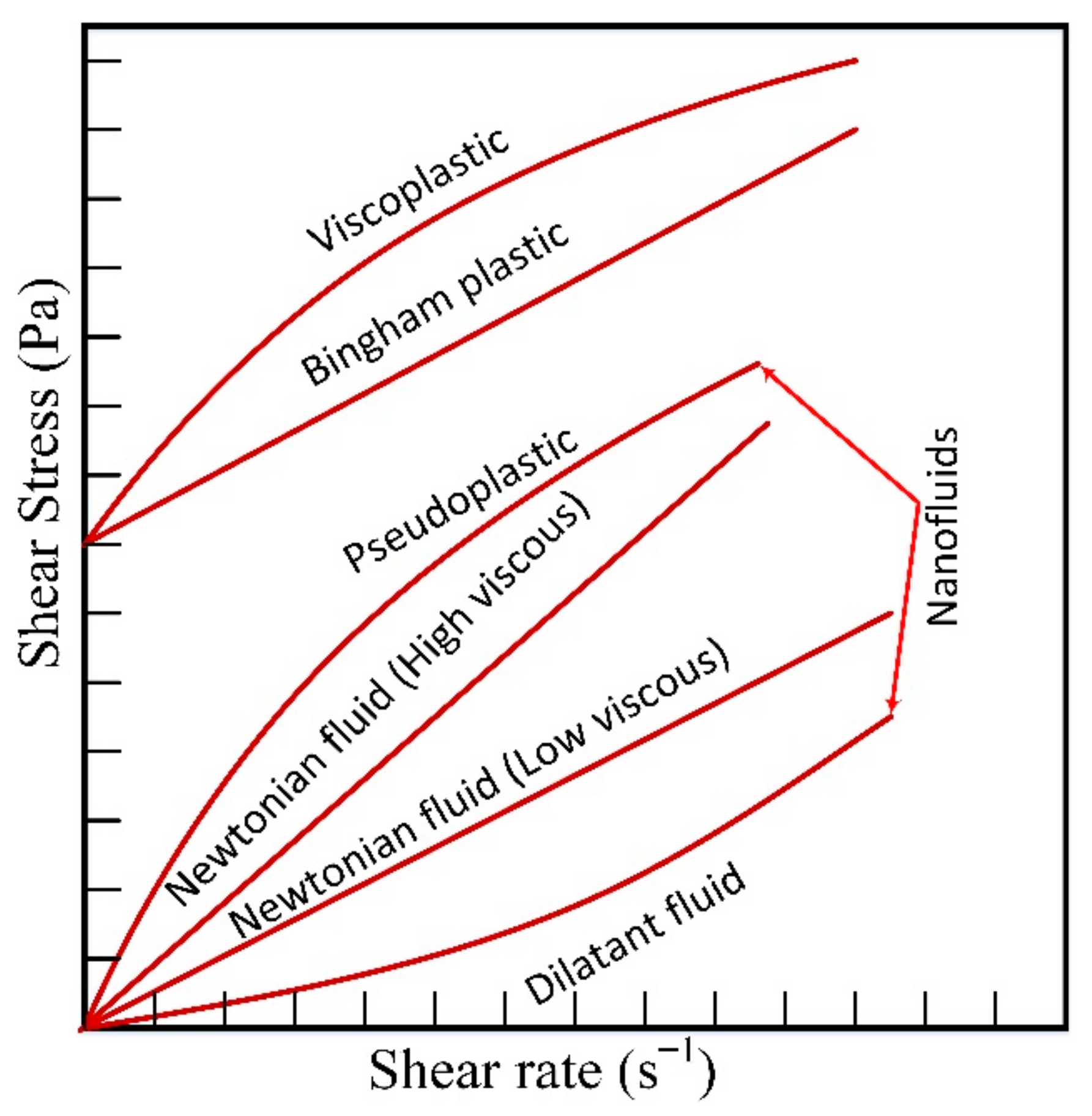
| Author | Nanoparticles | Base Fluid | Temperature-Range (°C) | Relative Viscosity (Maximum) | |
|---|---|---|---|---|---|
| Xichen et al. | [2636] | Al2O3 | Engine oil (SN 5W−40 | Ambient | 1.12 |
| Mostafizur et al. | [2737] | TiO2 | Methanol | 1–20 | 1.65 |
| Chiam et al. | [4238] | Al2O3 | 60:40 (W:EG) | 30–70 | 1.67 |
| Fedele et al. | [4339] | TiO2 | Bidistilled water | 10–70 | 2.8 |
| Sujith et al. | [9] | Al2O3 | Coconut oil | 30–140 | 2.5 |
| Georgiana et al. | [4440] | Al2O3/SiO2 | Distilled water | Ambient | 2.7 |
| Suhaib et al. | [4541] | ZnO | Paraffin oil | 25–55 | 1.62 |
| Yan et al. | [4642] | TiO2/MWCNT | Ethylene glycol | 25–55 | 1.94 |
| Kole et al. | [4743] | CuO | Gearoil | 10–80 | 2.8 |
| Andac et al. | [4844] | ZrO2 | Water | 10–70 | 1.8 |
| Sonawane | [ |
| Pak and Cho | |
| [ | 2319] |
|
A, B and C are correlation coefficients |
Vajjha and Das |
| [ | 2420] |
| Zhou et al. | |
| [ | 2521] |
| Shekar and Sharma | |
| [ | 2622] |
| Donghyun and Debjyoti | |
| [ | 2723] |
2.2. Thermal Conductivity
3. Conclusions
References
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. Lund, H.; Werner, S.; Wiltshire, R.; Svendsen, S.; Thorsen, J.E.; Hvelplund, F.; Mathiesen, B.V. 4th Generation District Heating (4GDH). Integrating smart thermal grids into future sustainable energy systems. Energy 2014, 68, 1–11.
- Vajjha, R.S.; Das, D.K. Specific heat measurement of three nanofluids and development of new correlations. J. Heat Transf. 2009, 131, 071601. Godson, L.; Raja, B.; Lal, D.M.; Wongwises, S. Enhancement of heat transfer using nanofluids—An overview. Renew. Sustain. Energy Rev. 2010, 14, 629–641.
- Zhou, L.-P.; Wang, B.-X.; Peng, X.-F.; Du, X.-Z.; Yang, Y.-P. On the specific heat capacity of CuO nanofluid. Adv. Mech. Eng. 2010, 2010, 172085. Bég, O.A.; Espinoza, D.E.S.; Kadir, A.; Shamshuddin, M.; Sohail, A. Experimental study of improved rheology and lubricity of drilling fluids enhanced with nano-particles. Appl. Nanosci. 2018, 8, 1069–1090.
- Sekhar, Y.R.; Sharma, K.V. Study of viscosity and specific heat capacity characteristics of water-based Al2O3 nanofluids at low particle concentrations. J. Exp. Nanosci. 2013, 10, 86–102. Sriharan, G.; Harikrishnan, S.; Ali, H.M. Experimental investigation on the effectiveness of MHTHS using different metal oxide-based nanofluids. J. Therm. Anal. 2021, 143, 1251–1260.
- Shin, D.; Banerjee, D. Specific heat of nanofluids synthesized by dispersing alumina nanoparticles in alkali salt eutectic. Int. J. Heat Mass Transf. 2014, 74, 210–214. Jamshed, W.; Şirin, C.; Selimefendigil, F.; Shamshuddin, M.D.; Altowairqi, Y.; Eid, M.R. Thermal Characterization of Coolant Maxwell Type Nanofluid Flowing in Parabolic Trough Solar Collector (PTSC) Used Inside Solar Powered Ship Application. Coatings 2021, 11, 1552.
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Cambridge University Press: Cambridge, UK, 1873. Chandrasekar, M.; Suresh, S.; Bose, A.C. Experimental studies on heat transfer and friction factor characteristics of Al2O3/water nanofluid in a circular pipe under laminar flow with wire coil inserts. Exp. Therm. Fluid Sci. 2010, 34, 122–130.
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer konstanten von heterogenen substanzen. I. dielektrizitätskonstanten und leitfähigkeiten der mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced thermal conductivity of TiO2—Water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373.
- Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 2009, 106, 014304. Koshy, C.P.; Rajendrakumar, P.K.; Thottackkad, M.V. Evaluation of the tribological and thermo-physical properties of coconut oil added with MoS 2 nanoparticles at elevated temperatures. Wear 2015, 330, 288–308.
- Sujith, S.V.; Solanki, A.K.; Mulik, R.S. Experimental evaluation in thermal conductivity enhancement and heat transfer optimization of eco-friendly Al2O3–pure coconut oil based nano fluids. J. Therm. Sci. Eng. Appl. 2021, 13, 1–13. Sujith, S.; Solanki, A.K.; Mulik, R.S. Experimental evaluation on rheological behavior of Al2O3-pure coconut oil nanofluids. J. Mol. Liq. 2019, 286, 110905.
- Yu, W.; Choi, S.U.S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171. Esfe, M.H.; Arani, A.A.A.; Esfandeh, S. Experimental study on rheological behavior of monograde heavy-duty engine oil containing CNTs and oxide nanoparticles with focus on viscosity analysis. J. Mol. Liq. 2018, 272, 319–329.
- Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. Nguyen, C.; Desgranges, F.; Roy, G.; Galanis, N.; Maré, T.; Boucher, S.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids—Hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506.
- Koo, J.; Kleinstreuer, C. A new thermal conductivity model for nanofluids. J. Nanopart. Res. 2004, 6, 577–588. Nadooshan, A.A.; Esfe, M.H.; Afrand, M. Evaluation of rheological behavior of 10W40 lubricant containing hybrid nano-material by measuring dynamic viscosity. Phys. E Low Dimens. Syst. Nanostruct. 2017, 92, 47–54.
- Khdher, A.M.; Sidik, N.A.C.; Hamzah, W.A.W.; Mamat, R. An experimental determination of thermal conductivity and electrical conductivity of bio glycol based Al2O3 nanofluids and development of new correlation. Int. Commun. Heat Mass Transf. 2016, 73, 75–83. Maxwell, J.C. A Treatise on Electricity and Magnetism; Cambridge University Press: Cambridge, UK, 1873.
- Lu, S.; Lin, H. Effective conductivity of composites containing aligned spheroidal inclusions of finite conductivity. J. Appl. Phys. 1996, 79, 6761–6769. Choi, J.E.S. Enhancing Thermal Conductivity of Fluids with Nanoparticles. 1995. Available online: https://www.osti.gov/biblio/196525-enhancing-thermal-conductivity-fluids-nanoparticles (accessed on 10 December 2021).
- Kim, S.H.; Choi, S.R.; Kim, D. Thermal conductivity of metal-oxide nanofluids: Particle size dependence and effect of laser irradiation. J. Heat Transf. 2007, 129, 298–307. Kim, S.H.; Choi, S.R.; Kim, D. Thermal conductivity of metal-oxide nanofluids: Particle size dependence and effect of laser irradiation. J. Heat Transf. 2007, 129, 298–307.
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubir, M.N.B.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15. Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubir, M.N.B.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15.
- Qi, C.; Luo, T.; Liu, M.; Fan, F.; Yan, Y. Experimental study on the flow and heat transfer characteristics of nanofluids in double-tube heat exchangers based on thermal efficiency assessment. Energy Convers. Manag. 2019, 197, 111877. Qi, C.; Luo, T.; Liu, M.; Fan, F.; Yan, Y. Experimental study on the flow and heat transfer characteristics of nanofluids in double-tube heat exchangers based on thermal efficiency assessment. Energy Convers. Manag. 2019, 197, 111877.
- Xuan, Y.; Li, Q. Investigation on convective heat transfer and flow features of nanofluids. J. Heat Transf. 2003, 125, 151–155. Xuan, Y.; Li, Q. Investigation on convective heat transfer and flow features of nanofluids. J. Heat Transf. 2003, 125, 151–155.
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170.
- Vajjha, R.S.; Das, D.K. Specific heat measurement of three nanofluids and development of new correlations. J. Heat Transf. 2009, 131, 071601. Vajjha, R.S.; Das, D.K. Specific heat measurement of three nanofluids and development of new correlations. J. Heat Transf. 2009, 131, 071601.
- Zhou, L.-P.; Wang, B.-X.; Peng, X.-F.; Du, X.-Z.; Yang, Y.-P. On the specific heat capacity of CuO nanofluid. Adv. Mech. Eng. 2010, 2010, 172085. Zhou, L.-P.; Wang, B.-X.; Peng, X.-F.; Du, X.-Z.; Yang, Y.-P. On the specific heat capacity of CuO nanofluid. Adv. Mech. Eng. 2010, 2010, 172085.
- Sekhar, Y.R.; Sharma, K.V. Study of viscosity and specific heat capacity characteristics of water-based Al2O3 nanofluids at low particle concentrations. J. Exp. Nanosci. 2013, 10, 86–102. Sekhar, Y.R.; Sharma, K.V. Study of viscosity and specific heat capacity characteristics of water-based Al2O3 nanofluids at low particle concentrations. J. Exp. Nanosci. 2013, 10, 86–102.
- Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. Shin, D.; Banerjee, D. Specific heat of nanofluids synthesized by dispersing alumina nanoparticles in alkali salt eutectic. Int. J. Heat Mass Transf. 2014, 74, 210–214.
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer konstanten von heterogenen substanzen. I. dielektrizitätskonstanten und leitfähigkeiten der mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191.
- Akita, O.; Yamada, E. Effective thermal conductivity of dispersed materials. Heat Mass Transf. 1980, 13, 27–37. Bruggeman, D.A.G. Berechnung verschiedener physikalischer konstanten von heterogenen substanzen. I. dielektrizitätskonstanten und leitfähigkeiten der mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664.
- Sujith, S.V.; Solanki, A.K.; Mulik, R.S. Experimental evaluation in thermal conductivity enhancement and heat transfer optimization of eco-friendly Al2O3–pure coconut oil based nano fluids. J. Therm. Sci. Eng. Appl. 2021, 13, 1–13. Akita, O.; Yamada, E. Effective thermal conductivity of dispersed materials. Heat Mass Transf. 1980, 13, 27–37.
- Yu, W.; Choi, S.U.S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171. Sujith, S.V.; Solanki, A.K.; Mulik, R.S. Experimental evaluation in thermal conductivity enhancement and heat transfer optimization of eco-friendly Al2O3–pure coconut oil based nano fluids. J. Therm. Sci. Eng. Appl. 2021, 13, 1–13.
- Krieger, I.M.; Dougherty, T.J. A mechanism for non-newtonian flow in suspensions of rigid spheres. Trans. Soc. Rheol. 1959, 3, 137–152. Yu, W.; Choi, S.U.S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171.
- Chhabra, R.P. Non-newtonian fluids: An introduction. In Rheolology Complex Fluids; Springer: New York, NY, USA, 2010; pp. 3–34. Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 2009, 106, 014304.
- Esfe, M.H.; Rostamian, H. Non-Newtonian power-law behavior of TiO2 /SAE 50 nano-lubricant: An experimental report and new correlation. J. Mol. Liq. 2017, 232, 219–225. Koo, J.; Kleinstreuer, C. A new thermal conductivity model for nanofluids. J. Nanopart. Res. 2004, 6, 577–588.
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Rheological behaviour of nanofluids: A review. Renew. Sustain. Energy Rev. 2015, 53, 779–791. Khdher, A.M.; Sidik, N.A.C.; Hamzah, W.A.W.; Mamat, R. An experimental determination of thermal conductivity and electrical conductivity of bio glycol based Al2O3 nanofluids and development of new correlation. Int. Commun. Heat Mass Transf. 2016, 73, 75–83.
- Hu, X.; Yin, D.; Chen, X.; Xiang, G. Experimental investigation and mechanism analysis: Effect of nanoparticle size on viscosity of nanofluids. J. Mol. Liq. 2020, 314, 113604. Lu, S.; Lin, H. Effective conductivity of composites containing aligned spheroidal inclusions of finite conductivity. J. Appl. Phys. 1996, 79, 6761–6769.
- Mostafizur, R.; Aziz, A.A.; Saidur, R.; Bhuiyan, M.; Mahbubul, I. Effect of temperature and volume fraction on rheology of methanol based nanofluids. Int. J. Heat Mass Transf. 2014, 77, 765–769. Chhabra, R.P. Non-newtonian fluids: An introduction. In Rheolology Complex Fluids; Springer: New York, NY, USA, 2010; pp. 3–34.
- Chiam, H.; Azmi, W.; Usri, N.; Mamat, R.; Adam, N. Thermal conductivity and viscosity of Al2O3 nanofluids for different based ratio of water and ethylene glycol mixture. Exp. Therm. Fluid Sci. 2017, 81, 420–429. Esfe, M.H.; Rostamian, H. Non-Newtonian power-law behavior of TiO2 /SAE 50 nano-lubricant: An experimental report and new correlation. J. Mol. Liq. 2017, 232, 219–225.
- Fedele, L.; Colla, L.; Bobbo, S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366. Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Rheological behaviour of nanofluids: A review. Renew. Sustain. Energy Rev. 2015, 53, 779–791.
- Moldoveanu, G.M.; Ibanescu, C.; Danu, M.; Minea, A.A. Viscosity estimation of Al2O3, SiO2 nanofluids and their hybrid: An experimental study. J. Mol. Liq. 2018, 253, 188–196. Hu, X.; Yin, D.; Chen, X.; Xiang, G. Experimental investigation and mechanism analysis: Effect of nanoparticle size on viscosity of nanofluids. J. Mol. Liq. 2020, 314, 113604.
- Ilyas, S.U.; Narahari, M.; Theng, J.T.Y.; Pendyala, R. Experimental evaluation of dispersion behavior, rheology and thermal analysis of functionalized zinc oxide-paraffin oil nanofluids. J. Mol. Liq. 2019, 294, 111613. Mostafizur, R.; Aziz, A.A.; Saidur, R.; Bhuiyan, M.; Mahbubul, I. Effect of temperature and volume fraction on rheology of methanol based nanofluids. Int. J. Heat Mass Transf. 2014, 77, 765–769.
- Yan, S.-R.; Kalbasi, R.; Nguyen, Q.; Karimipour, A. Rheological behavior of hybrid MWCNTs-TiO2/EG nanofluid: A comprehensive modeling and experimental study. J. Mol. Liq. 2020, 308, 113058. Chiam, H.; Azmi, W.; Usri, N.; Mamat, R.; Adam, N. Thermal conductivity and viscosity of Al2O3 nanofluids for different based ratio of water and ethylene glycol mixture. Exp. Therm. Fluid Sci. 2017, 81, 420–429.
- Kole, M.; Dey, T. Effect of aggregation on the viscosity of copper oxide—gear oil nanofluids. Int. J. Therm. Sci. 2011, 50, 1741–1747. Fedele, L.; Colla, L.; Bobbo, S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366.
- Çolak, A.B. A novel comparative analysis between the experimental and numeric methods on viscosity of zirconium oxide nanofluid: Developing optimal artificial neural network and new mathematical model. Powder Technol. 2021, 381, 338–351. Moldoveanu, G.M.; Ibanescu, C.; Danu, M.; Minea, A.A. Viscosity estimation of Al2O3, SiO2 nanofluids and their hybrid: An experimental study. J. Mol. Liq. 2018, 253, 188–196.
- Sonawane, S.S.; Juwar, V. Optimization of conditions for an enhancement of thermal conductivity and minimization of viscosity of ethylene glycol based Fe3O4 nanofluid. Appl. Therm. Eng. 2016, 109, 121–129. Ilyas, S.U.; Narahari, M.; Theng, J.T.Y.; Pendyala, R. Experimental evaluation of dispersion behavior, rheology and thermal analysis of functionalized zinc oxide-paraffin oil nanofluids. J. Mol. Liq. 2019, 294, 111613.
- Boubaker, R.; Harmand, S.; Platel, V. Numerical analysis of the impact of nanofluids and vapor grooves design on the performance of capillary evaporators. Transp. Porous Media 2018, 122, 401–419. Yan, S.-R.; Kalbasi, R.; Nguyen, Q.; Karimipour, A. Rheological behavior of hybrid MWCNTs-TiO2/EG nanofluid: A comprehensive modeling and experimental study. J. Mol. Liq. 2020, 308, 113058.
- Sarafraz, M.; Pourmehran, O.; Yang, B.; Arjomandi, M. Assessment of the thermal performance of a thermosyphon heat pipe using zirconia-acetone nanofluids. Renew. Energy 2019, 136, 884–895. Kole, M.; Dey, T. Effect of aggregation on the viscosity of copper oxide—gear oil nanofluids. Int. J. Therm. Sci. 2011, 50, 1741–1747.
- Mebarek-Oudina, F. Convective heat transfer of Titania nanofluids of different base fluids in cylindrical annulus with discrete heat source. Heat Transf. Asian Res. 2019, 48, 135–147. Çolak, A.B. A novel comparative analysis between the experimental and numeric methods on viscosity of zirconium oxide nanofluid: Developing optimal artificial neural network and new mathematical model. Powder Technol. 2021, 381, 338–351.
- Sarafraz, M.; Hormozi, F. Pool boiling heat transfer to dilute copper oxide aqueous nanofluids. Int. J. Therm. Sci. 2015, 90, 224–237. Sonawane, S.S.; Juwar, V. Optimization of conditions for an enhancement of thermal conductivity and minimization of viscosity of ethylene glycol based Fe3O4 nanofluid. Appl. Therm. Eng. 2016, 109, 121–129.
- Singh, T.; Dureja, J.S.; Dogra, M.; Bhatti, M.S. Environment friendly machining of inconel 625 under nano-fluid minimum quantity lubrication (NMQL). Int. J. Precis. Eng. Manuf. 2018, 19, 1689–1697. Boubaker, R.; Harmand, S.; Platel, V. Numerical analysis of the impact of nanofluids and vapor grooves design on the performance of capillary evaporators. Transp. Porous Media 2018, 122, 401–419.
- Behera, B.C.; Chetan; Setti, D.; Ghosh, S.; Rao, P.V. Spreadability studies of metal working fluids on tool surface and its impact on minimum amount cooling and lubrication turning. J. Mater. Process. Technol. 2017, 244, 1–16. Sarafraz, M.; Pourmehran, O.; Yang, B.; Arjomandi, M. Assessment of the thermal performance of a thermosyphon heat pipe using zirconia-acetone nanofluids. Renew. Energy 2019, 136, 884–895.
- Mebarek-Oudina, F. Convective heat transfer of Titania nanofluids of different base fluids in cylindrical annulus with discrete heat source. Heat Transf. Asian Res. 2019, 48, 135–147.
- Sarafraz, M.; Hormozi, F. Pool boiling heat transfer to dilute copper oxide aqueous nanofluids. Int. J. Therm. Sci. 2015, 90, 224–237.
- Singh, T.; Dureja, J.S.; Dogra, M.; Bhatti, M.S. Environment friendly machining of inconel 625 under nano-fluid minimum quantity lubrication (NMQL). Int. J. Precis. Eng. Manuf. 2018, 19, 1689–1697.
- Behera, B.C.; Chetan; Setti, D.; Ghosh, S.; Rao, P.V. Spreadability studies of metal working fluids on tool surface and its impact on minimum amount cooling and lubrication turning. J. Mater. Process. Technol. 2017, 244, 1–16.
- Chhabra, R.P. Non-newtonian fluids: An introduction. In Rheolology Complex Fluids; Springer: New York, NY, USA, 2010; pp. 3–34.
- Esfe, M.H.; Rostamian, H. Non-Newtonian power-law behavior of TiO2 /SAE 50 nano-lubricant: An experimental report and new correlation. J. Mol. Liq. 2017, 232, 219–225.
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Rheological behaviour of nanofluids: A review. Renew. Sustain. Energy Rev. 2015, 53, 779–791.
- Hu, X.; Yin, D.; Chen, X.; Xiang, G. Experimental investigation and mechanism analysis: Effect of nanoparticle size on viscosity of nanofluids. J. Mol. Liq. 2020, 314, 113604.
- Mostafizur, R.; Aziz, A.A.; Saidur, R.; Bhuiyan, M.; Mahbubul, I. Effect of temperature and volume fraction on rheology of methanol based nanofluids. Int. J. Heat Mass Transf. 2014, 77, 765–769.
- Chiam, H.; Azmi, W.; Usri, N.; Mamat, R.; Adam, N. Thermal conductivity and viscosity of Al2O3 nanofluids for different based ratio of water and ethylene glycol mixture. Exp. Therm. Fluid Sci. 2017, 81, 420–429.
- Fedele, L.; Colla, L.; Bobbo, S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366.
- Moldoveanu, G.M.; Ibanescu, C.; Danu, M.; Minea, A.A. Viscosity estimation of Al2O3, SiO2 nanofluids and their hybrid: An experimental study. J. Mol. Liq. 2018, 253, 188–196.
- Ilyas, S.U.; Narahari, M.; Theng, J.T.Y.; Pendyala, R. Experimental evaluation of dispersion behavior, rheology and thermal analysis of functionalized zinc oxide-paraffin oil nanofluids. J. Mol. Liq. 2019, 294, 111613.
- Yan, S.-R.; Kalbasi, R.; Nguyen, Q.; Karimipour, A. Rheological behavior of hybrid MWCNTs-TiO2/EG nanofluid: A comprehensive modeling and experimental study. J. Mol. Liq. 2020, 308, 113058.
- Kole, M.; Dey, T. Effect of aggregation on the viscosity of copper oxide—gear oil nanofluids. Int. J. Therm. Sci. 2011, 50, 1741–1747.
- Çolak, A.B. A novel comparative analysis between the experimental and numeric methods on viscosity of zirconium oxide nanofluid: Developing optimal artificial neural network and new mathematical model. Powder Technol. 2021, 381, 338–351.
- Sonawane, S.S.; Juwar, V. Optimization of conditions for an enhancement of thermal conductivity and minimization of viscosity of ethylene glycol based Fe3O4 nanofluid. Appl. Therm. Eng. 2016, 109, 121–129.
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Cambridge University Press: Cambridge, UK, 1873.
- Choi, J.E.S. Enhancing Thermal Conductivity of Fluids with Nanoparticles. 1995. Available online: https://www.osti.gov/biblio/196525-enhancing-thermal-conductivity-fluids-nanoparticles (accessed on 10 December 2021).
- Kim, S.H.; Choi, S.R.; Kim, D. Thermal conductivity of metal-oxide nanofluids: Particle size dependence and effect of laser irradiation. J. Heat Transf. 2007, 129, 298–307.
- Boubaker, R.; Harmand, S.; Platel, V. Numerical analysis of the impact of nanofluids and vapor grooves design on the performance of capillary evaporators. Transp. Porous Media 2018, 122, 401–419.
- Sarafraz, M.; Pourmehran, O.; Yang, B.; Arjomandi, M. Assessment of the thermal performance of a thermosyphon heat pipe using zirconia-acetone nanofluids. Renew. Energy 2019, 136, 884–895.
- Mebarek-Oudina, F. Convective heat transfer of Titania nanofluids of different base fluids in cylindrical annulus with discrete heat source. Heat Transf. Asian Res. 2019, 48, 135–147.
- Sarafraz, M.; Hormozi, F. Pool boiling heat transfer to dilute copper oxide aqueous nanofluids. Int. J. Therm. Sci. 2015, 90, 224–237.
- Singh, T.; Dureja, J.S.; Dogra, M.; Bhatti, M.S. Environment friendly machining of inconel 625 under nano-fluid minimum quantity lubrication (NMQL). Int. J. Precis. Eng. Manuf. 2018, 19, 1689–1697.
- Behera, B.C.; Chetan; Setti, D.; Ghosh, S.; Rao, P.V. Spreadability studies of metal working fluids on tool surface and its impact on minimum amount cooling and lubrication turning. J. Mater. Process. Technol. 2017, 244, 1–16.