Fluorescence microscopy has become a critical tool for researchers to understand biological processes at the cellular level. Micrographs from fixed and live-cell imaging procedures feature in a plethora of scientific articles for the field of cell biology.
- luorescence microscopy
- microscopy techniques
- imaging agents
- cellular imaging
1. Introduction
2. Fluorescence Microscope Hardware Systems
2.1. Multiphoton and Other Advanced Microscopy Techniques
Modifying the laser excitation source of a confocal microscope with an ultra-fast pulsed laser enables two- or three-photon microscopy [5]. Ultra-fast lasers allow deeper penetration of samples using longer excitation wavelengths, and optical sections are created through confinement of the two (or multi) photon effect to a single focal plane. This technique is suited to imaging large tissue samples, where penetration depths of 100 μm or more are needed to investigate macro-scale biological processes. Multiphoton microscopes are also needed for intravital imaging, where both gentle illumination and imaging depth are critical for imaging live whole organisms. However, multiphoton microscopes are limited by the added expense of purchasing an additional ultra-fast laser, which can add significantly to the cost of ownership and operation. Laser scanning microscopes have been adapted to observe dynamic events in cellular function and exploit the properties of imaging probes, such as fluorescence lifetimes, and their interactions within and between cells. The three most common technologies employed are fluorescence lifetime imaging microscopy (FLIM) [6], fluorescence resonance energy transfer (FRET) [7], and fluorescence recovery after photobleaching (FRAP) [6]. FLIM requires the observation of intensity changes in a fluorophore over a course of time, which has been useful for quantifying decay rates of cell metabolites [8]. FRET measures energy transfer from one fluorophore to another using a single excitation, which allows for a variety of applications, such as calcium imaging [9] and protein–protein interactions/transfers [10]. Finally, FRAP measures the recovery in signal of a molecular probe after photobleaching, which enables the observation of diffusion kinetics in both cells and tissue samples [11]. Another recent technique, used to image larger tissue samples quickly and efficiently, is light-sheet fluorescence microscopy (LSFM) [12]. Light-sheet microscope operation differs from confocal microscopy, as samples are excited using a sheet of light from the side, as opposed to a focussed beam of light from the top or bottom of a sample. Emitted light is then detected perpendicular to the excitation sheet, unlike a confocal microscope, which detects the emitted signal in the opposite direction to the excitation beam. The thickness of the sheet of light determines the thickness of the optical section captured, and the sample can be rotated and moved through this plane to produce a three-dimensional tomographic image. Light-sheet fluorescence microscopy enables imaging of larger, multicellular samples, such as organoids and whole organisms (e.g., insects, plants, and animals [13][14][15]) that may not be adequately captured in a timely manner using single or multi-photon microscopy, although this does require tissue-clearing methodology to create optically transparent samples [16][17][18].2.2. Super Resolution Microscopy (SRM); Going beyond the Limits of Light
Improvements in laser scanning microscopes have enhanced imaging resolutions beyond Abbe’s limit (Equation (1)), the physical limitation due to the physics of diffraction, for example, a 200 nm resolution limit in air when using an excitation wavelength of 400 nm [19]; however, compromises such as hardware cost, phototoxicity, and low capture speed, remain limitations. The simplest technique to improve resolution using a confocal microscope is to restrict pinhole size, perform a z-stack, which involves combining multiple images captured at sequential focal planes, and perform post-processing of images using software deconvolution. However, acquiring a suitable z-stack of images involves repeated laser excitation, which significantly increases both cellular phototoxicity and the time to capture the complete micrograph [20].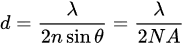
3. Biological Models for Fluorescence Imaging
3.1. Monolayer Cell Culture
There are over 4000 well-characterised, readily available cell lines serving as models for human disease and development, which can be studied by researchers using a variety of imaging applications. Cell culture systems provide several advantages as models of more complex biological systems, including their potential for high-throughput screening, reproducibility, cost-effectiveness, reduced ethical considerations, well-documented protocols, and their ability to be easily manipulated. For example, cells can be transiently or stably transfected to introduce a gene of interest, enabling visualisation of a given protein using fluorescent protein tags. Cell lines offer an isolated monoculture of a single cell type, or co-culture of multiple cell types, which typically takes the form of a thin adherent monolayer or suspension culture [24][25]. For adherent cells, a uniform monolayer permits improved light penetration for imaging and consistent staining/immunolabelling, without the need to permeate dyes and antibodies into deeper layers of a sample. This reduces sample-sample variability, providing more reproducible imaging results. Moreover, cultured cells exhibit less endogenous fluorescence, which can interfere with label detection, compared to processed tissues and organs [26], and do not require time-consuming tissue processing for imaging. A microscope equipped with an incubation system enables real-time visualisation of live cells [27] and time-lapse imaging [16], with minimal interference to normal cellular function due to environmental disturbance. Microscopy using cell line models enables the exploration of subcellular processes that are implicated in disease pathogenesis, as well as cellular responses to therapeutics [28][29][30]. Whole cell imaging of two-dimensional (2D) cultures allows the examination of cell morphology [31] and intercellular communication networks, mediated by structures such as filopodia or cytonemes [32], tunnelling nanotubes [33], and cellular bridges [34]. Live cell imaging of cell cultures also enables the study of dynamic cell behaviour, such as extracellular vesicle formation, cell motility and migration in wound healing [35], and cancer metastasis [29]. Fluorescence microscopy of cell culture is also widely used to image subcellular components, including organelles and molecules, to provide greater insight into their structure, function, and subcellular localisation. Recent examples of the impressive details of cellular structures visualised by super-resolution microscopy (SRM) include nuclear pore complex organisation, membrane-associated periodic skeleton in neurons and synaptic structures [36]. Multispectral imaging, using confocal and lattice light-microscopy, revealed an intricate spatial-temporal organelle interactome in live cells [37], illustrating the usefulness of novel microscopy techniques to better understand complex cell biology.3.2. 3D Cell Cultures
The need for animal-free disease models that avoid the limitations of traditional cell culture monolayers, yet better mimic human in vivo environments and recapitulate the complex interactions between different cell types, has resulted in advances in 3D cell culture models. These can represent many tissues, including brain [38], breast [39], and prostate tissue [40], utilising scaffold or scaffold-free techniques to induce or cultivate their formation or maintenance. There are multiple types of 3D cultures, ranging from spheroids of cell lines to organoids derived from patient tissue (reviewed by Caleb and Yong [41]). Indeed, patient-derived organoids are utilised to offer personalised treatments and novel therapeutic discoveries (e.g., [42][43]). Generated from cell lines cultures, spheroids or organoids may preserve the cell phenotypes observed in tissues, which result from interactions and responses to the microenvironment, such as detecting changes to necrotic tissue due to nutrient starvation in the centre of spheroids, mimicking rapidly growing non-angiogenic tumour tissue [44]. An example of the differences between 2D and 3D culture was demonstrated in cardiac cells; cells grown in 2D exhibited a large network of microfilaments and microtubules, whilst cells in a 3D environment were smaller in size, had many junctions between cells, and exhibited increased alpha actinin cytoskeletal protein [45]. Thus, 3D cell cultures may provide significant new insights into more complex cell biology, as well as disease biomarkers and therapeutics to improve translation.3.3. Tissue Sections
A truer representation of in vivo biology is of course to take it directly from the source: tissue. The major advantage of using tissue sections compared to cell line models is that the complex interactions between a cell with its microenvironment are preserved, including the presence of the supporting extracellular matrix [46], the influence of stroma and immune cells [47], and the maintenance of cell polarity in the hierarchical architecture of the tissue [48]. The ability to collect a snapshot of in vivo biology and preserve this for future study is invaluable, and biobanks with archived tissue exist for a multitude of diseases.3.4. Intravital Imaging
Intravital imaging requires access to a high-resolution confocal, multiphoton, and/or light-sheet microscope, and the development of suitable animal models. Transgenic animals modified to express fluorescently tagged proteins provide a true in vivo environment for evaluating various cellular processes, with constitutive replenishment of fluorescently labelled proteins enabling long-term tracing in a cell- and tissue-specific manner. This technique mainly makes use of small organisms, such as fruit flies [64][65][66], zebrafish [53][67][68], and mice [69][70], due to their short generation times, established genetic lines, and relatively low cost. Subcutaneous and orthotopic xenograft models used in intravital studies can accurately provide insights into tumour heterogeneity and responses to drug treatments [71][72]. With the use of intravital microscopy, it has also become possible to visualise the inter-individual variability at a microscopic level in response to drug treatment. An advantage of intravital imaging is the continual monitoring of physiological changes over days and weeks, which is especially important in developmental cells [55], stem cell [73][74], and tumour biology [75].
4. Imaging Agents Used for Fluorescence Microscopy
4.1. Fluorescent Proteins
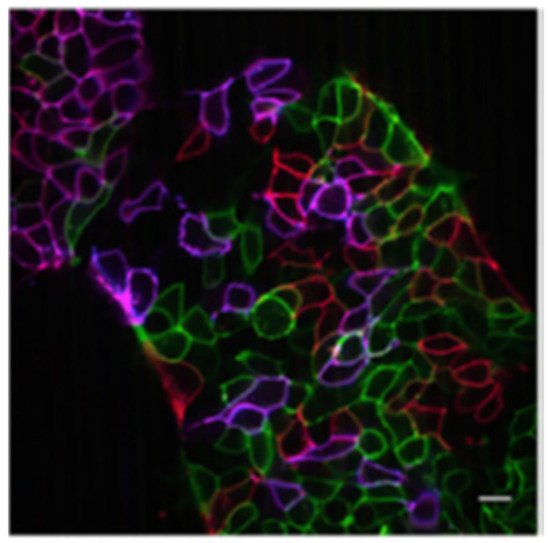
4.2. Graphene Quantum Dots
4.3. Metal Ion Complexes
Metal ion complexes have been successfully used in cellular imaging applications such as subcellular compartment staining and visualisation of cellular processes. A variety of sensitising pathways have been utilised for these types of metal complexes, such as metal-to-ligand charge transfer (MLCT) and ligand-to-ligand charge transfer (LLCT), which have been reviewed previously [101]. The major advantage of using metal ion complexes as imaging agents are their long-lived emission profiles, which facilitate the use of time-gated fluorescence microscopy experiments, such as FLIM, and enables the visualisation of cellular events, which is otherwise not possible due to endogenous autofluorescence within cells [102]. Moreover, metal ion complexes offer highly tuneable excitation and emission profiles, which span the entire visible and near-IR spectrum. They also offer the largest Stokes shifts of the four classes covered in this review (typically greater than 5000 cm−1), which obviates self-quenching issues encountered by most organic fluorophores [103].4.4. Organic Fluorophores
Organic fluorophores enjoy a privileged place as the most prominent class of compounds for fluorescence imaging. Owing to their ready accessibility, small size, wide variety, and excellent emissive properties, organic fluorophores are frequently used for imaging cellular components, visualising cellular processes and tagging larger molecules (including antibodies and drugs) for insights into their cellular activity. The advantages and applications of organic fluorophores have been thoroughly covered in many excellent revstudiews [104][105][106][107][108], and their commercial availability renders their use as “plug and play” for cell biologists.References
- Renz, M. Fluorescence microscopy—A historical and technical perspective. Cytom. Part A 2013, 83, 767–779.
- Dunst, S.; Tomancak, P. Imaging flies by fluorescence microscopy: Principles, technologies, and applications. Genetics 2018, 211, 15–34.
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green fluorescent protein as a marker for gene expression. Science 1994, 263, 802–805.
- Prasher, D.C.; Eckenrode, V.K.; Ward, W.W.; Prendergast, F.G.; Cormier, M.J. Primary structure of the aequorea victoria green-fluorescent protein. Gene 1992, 111, 229–233.
- Murphy, D.B.; Michael, D. Two-photon excitation fluorescence microscopy. In Fundamentals of Light Microscopy and Electronic Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 307–329.
- Ishikawa-Ankerhold, H.C.; Ankerhold, R.; Drummen, G.P.C. Advanced fluorescence microscopy techniques—Frap, flip, flap, fret and flim. Molecules 2012, 17, 4047–4132.
- De Los Santos, C.; Chang, C.-W.; Mycek, M.-A.; Cardullo, R.A. Frap, flim, and fret: Detection and analysis of cellular dynamics on a molecular scale using fluorescence microscopy. Mol. Reprod. Dev. 2015, 82, 587–604.
- Blacker, T.S.; Mann, Z.F.; Gale, J.E.; Ziegler, M.; Bain, A.J.; Szabadkai, G.; Duchen, M.R. Separating nadh and nadph fluorescence in live cells and tissues using flim. Nat. Commun. 2014, 5, 3936.
- Sekar, R.B.; Periasamy, A. Fluorescence resonance energy transfer (fret) microscopy imaging of live cell protein localizations. J. Cell Biol. 2003, 160, 629–633.
- Margineanu, A.; Chan, J.J.; Kelly, D.J.; Warren, S.C.; Flatters, D.; Kumar, S.; Katan, M.; Dunsby, C.W.; French, P.M.W. Screening for protein-protein interactions using förster resonance energy transfer (fret) and fluorescence lifetime imaging microscopy (flim). Sci. Rep. 2016, 6, 28186.
- Blumenthal, D.; Goldstien, L.; Edidin, M.; Gheber, L.A. Universal approach to frap analysis of arbitrary bleaching patterns. Sci. Rep. 2015, 5, 11655.
- Ji, N.; Magee, J.C.; Betzig, E. High-speed, low-photodamage nonlinear imaging using passive pulse splitters. Nat. Methods 2008, 5, 197–202.
- Sakaguchi, H.; Ozaki, Y.; Ashida, T.; Matsubara, T.; Oishi, N.; Kihara, S.; Takahashi, J. Self-organized synchronous calcium transients in a cultured human neural network derived from cerebral organoids. Stem Cell Rep. 2019, 13, 458–473.
- Au-Strobl, F.; Au-Klees, S.; Au-Stelzer, E.H.K. Light sheet-based fluorescence microscopy of living or fixed and stained tribolium castaneum embryos. JoVE 2017, 122, e55629.
- Ovečka, M.; von Wangenheim, D.; Tomančák, P.; Šamajová, O.; Komis, G.; Šamaj, J. Multiscale imaging of plant development by light-sheet fluorescence microscopy. Nat. Plants 2018, 4, 639–650.
- Chakraborty, T.; Driscoll, M.K.; Jeffery, E.; Murphy, M.M.; Roudot, P.; Chang, B.-J.; Vora, S.; Wong, W.M.; Nielson, C.D.; Zhang, H.; et al. Light-sheet microscopy of cleared tissues with isotropic, subcellular resolution. Nat. Methods 2019, 16, 1109–1113.
- Ueda, H.R.; Dodt, H.-U.; Osten, P.; Economo, M.N.; Chandrashekar, J.; Keller, P.J. Whole-brain profiling of cells and circuits in mammals by tissue clearing and light-sheet microscopy. Neuron 2020, 106, 369–387.
- Chen, B.-C.; Legant, W.R.; Wang, K.; Shao, L.; Milkie, D.E.; Davidson, M.W.; Janetopoulos, C.; Wu, X.S.; Hammer, J.A.; Liu, Z.; et al. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science 2014, 346, 1257998.
- Abbe, E. Contributions to the theory of the microscope and that microscopic perception. Arch. Microsc. Anat. 1873, 9, 413–468.
- Perez, V.; Chang, B.-J.; Stelzer, E.H.K. Optimal 2d-sim reconstruction by two filtering steps with richardson-lucy deconvolution. Sci. Rep. 2016, 6, 37149.
- Vicidomini, G.; Bianchini, P.; Diaspro, A. Sted super-resolved microscopy. Nat. Methods 2018, 15, 173–182.
- Huff, J. The airyscan detector from zeiss: Confocal imaging with improved signal-to-noise ratio and super-resolution. Nat. Methods 2015, 12.
- Wu, X.; Hammer, J.A. Zeiss airyscan: Optimizing usage for fast, gentle, super-resolution imaging. In Confocal Microscopy: Methods and Protocols; Brzostowski, J., Sohn, H., Eds.; Springer US: New York, NY, USA, 2021; pp. 111–130.
- Verma, A.; Verma, M.; Singh, A. Animal tissue culture principles and applications. Anim. Biotechnol. 2020, 269–293.
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer cell lines are useful model systems for medical research. Cancers 2019, 11, 1098.
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2005; Volume 11, pp. 227–256.
- Wang, C.; Han, B.; Zhou, R.; Zhuang, X. Real-time imaging of translation on single mrna transcripts in live cells. Cell 2016, 165, 990–1001.
- Bandaria, J.N.; Qin, P.; Berk, V.; Chu, S.; Yildiz, A. Shelterin protects chromosome ends by compacting telomeric chromatin. Cell 2016, 164, 735–746.
- Carragher, N.O. Profiling distinct mechanisms of tumour invasion for drug discovery: Imaging adhesion, signalling and matrix turnover. Clin. Exp. Metastasis 2009, 26, 381–397.
- Johnson, I.R.D.; Parkinson-Lawrence, E.J.; Shandala, T.; Weigert, R.; Butler, L.M.; Brooks, D.A. Altered endosome biogenesis in prostate cancer has biomarker potential. Mol. Cancer Res. 2014, 12, 1851.
- Losavio, B.E.; Liang, Y.; Santamaría-Pang, A.; Kakadiaris, I.A.; Colbert, C.M.; Saggau, P. Live neuron morphology automatically reconstructed from multiphoton and confocal imaging data. J. Neurophysiol. 2008, 100, 2422–2429.
- Bodeen, W.J.; Marada, S.; Truong, A.; Ogden, S.K. A fixation method to preserve cultured cell cytonemes facilitates mechanistic interrogation of morphogen transport. Development (Camb. Engl.) 2017, 144, 3612–3624.
- Bhat, S.; Ljubojevic, N.; Zhu, S.; Fukuda, M.; Echard, A.; Zurzolo, C. Rab35 and its effectors promote formation of tunneling nanotubes in neuronal cells. Sci. Rep. 2020, 10, 16803.
- Zani, B.G.; Indolfi, L.; Edelman, E.R. Tubular bridges for bronchial epithelial cell migration and communication. PLoS ONE 2010, 5, e8930.
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015, 24, 1635–1647.
- Sigal, Y.M.; Zhou, R.; Zhuang, X. Visualizing and discovering cellular structures with super-resolution microscopy. Science 2018, 361, 880–887.
- Valm, A.M.; Cohen, S.; Legant, W.R.; Melunis, J.; Hershberg, U.; Wait, E.; Cohen, A.R.; Davidson, M.W.; Betzig, E.; Lippincott-Schwartz, J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 2017, 546, 162–167.
- Gopalakrishnan, J. The emergence of stem cell-based brain organoids: Trends and challenges. BioEssays 2019, 41, 1900011.
- Rosenbluth, J.M.; Schackmann, R.C.J.; Gray, G.K.; Selfors, L.M.; Li, C.M.-C.; Boedicker, M.; Kuiken, H.J.; Richardson, A.; Brock, J.; Garber, J.; et al. Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nat. Commun. 2020, 11, 1711.
- Drost, J.; Karthaus, W.R.; Gao, D.; Driehuis, E.; Sawyers, C.L.; Chen, Y.; Clevers, H. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 2016, 11, 347–358.
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2d to 3d cell culture? Front. Mol. Biosci. 2020, 7, 33.
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.-J.; Chun, S.-M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991.
- Loong, H.H.F.; Wong, A.M.; Chan, D.T.M.; Cheung, M.S.H.; Chow, C.; Ding, X.; Chan, A.K.Y.; Johnston, P.A.; Lau, J.Y.W.; Poon, W.S.; et al. Patient-derived tumor organoid predicts drugs response in glioblastoma: A step forward in personalized cancer therapy? J. Clin. Neurosci. 2020, 78, 400–402.
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Jeong, E.K.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Regulation of tumor progression by programmed necrosis. Oxidative Med. Cell. Longev. 2018, 2018, 3537471.
- Pontes Soares, C.; Midlej, V.; de Oliveira, M.E.; Benchimol, M.; Costa, M.L.; Mermelstein, C. 2d and 3d-organized cardiac cells shows differences in cellular morphology, adhesion junctions, presence of myofibrils and protein expression. PLoS ONE 2012, 7, e38147.
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27.
- Mahajan, U.M.; Langhoff, E.; Goni, E.; Costello, E.; Greenhalf, W.; Halloran, C.; Ormanns, S.; Kruger, S.; Boeck, S.; Ribback, S.; et al. Immune cell and stromal signature associated with progression-free survival of patients with resected pancreatic ductal adenocarcinoma. Gastroenterology 2018, 155, 1625–1639.e1622.
- Wang, X.; Dong, B.; Zhang, K.; Ji, Z.; Cheng, C.; Zhao, H.; Sheng, Y.; Li, X.; Fan, L.; Xue, W.; et al. E-cadherin bridges cell polarity and spindle orientation to ensure prostate epithelial integrity and prevent carcinogenesis in vivo. PLOS Genet. 2018, 14, e1007609.
- Mulligan, S.J.; MacVicar, B.A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 2004, 431, 195–199.
- Takano, T.; Tian, G.-F.; Peng, W.; Lou, N.; Libionka, W.; Han, X.; Nedergaard, M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006, 9, 260–267.
- Chen, Z.; Ross, J.L.; Hambardzumyan, D. Intravital 2-photon imaging reveals distinct morphology and infiltrative properties of glioblastoma-associated macrophages. Proc. Natl. Acad. Sci. USA 2019, 116, 14254–14259.
- Lau, D.; Garçon, F.; Chandra, A.; Lechermann, L.M.; Aloj, L.; Chilvers, E.R.; Corrie, P.G.; Okkenhaug, K.; Gallagher, F.A. Intravital imaging of adoptive t-cell morphology, mobility and trafficking following immune checkpoint inhibition in a mouse melanoma model. Front. Immunol. 2020, 11, 11514.
- Sugano, Y.; Siegfried, H.; Merkel, E.; Drummond, I.A. Novel transgenic lines to analyze renal glutathione redox potential in vivo. Zebrafish 2020, 17, 153–155.
- Brighton, H.E.; Angus, S.P.; Bo, T.; Roques, J.; Tagliatela, A.C.; Darr, D.; Karagoz, K.; Sciaky, N.; Gatza, M.; Sharpless, N.E.; et al. New mechanisms of resistance to mek inhibitors in melanoma revealed by intravital imaging. Cancer Res. 2018, 78, 542–557.
- Huang, Q.; Cohen, M.A.; Alsina, F.C.; Devlin, G.; Garrett, A.; McKey, J.; Havlik, P.; Rakhilin, N.; Wang, E.; Xiang, K.; et al. Intravital imaging of mouse embryos. Science 2020, 368, 181–186.
- Dondossola, E.; Alexander, S.; Holzapfel, B.M.; Filippini, S.; Starbuck, M.W.; Hoffman, R.M.; Navone, N.; De-Juan-Pardo, E.M.; Logothetis, C.J.; Hutmacher, D.W.; et al. Intravital microscopy of osteolytic progression and therapy response of cancer lesions in the bone. Sci. Transl. Med. 2018, 10, 452.
- Alieva, M.; Leidgens, V.; Riemenschneider, M.J.; Klein, C.A.; Hau, P.; van Rheenen, J. Intravital imaging of glioma border morphology reveals distinctive cellular dynamics and contribution to tumor cell invasion. Sci. Rep. 2019, 9, 2054.
- You, S.; Tu, H.; Chaney, E.J.; Sun, Y.; Zhao, Y.; Bower, A.J.; Liu, Y.-Z.; Marjanovic, M.; Sinha, S.; Pu, Y.; et al. Intravital imaging by simultaneous label-free autofluorescence-multiharmonic microscopy. Nat. Commun. 2018, 9, 2125.
- Seynhaeve, A.L.B.; Oostinga, D.; van Haperen, R.; Eilken, H.M.; Adams, S.; Adams, R.H.; ten Hagen, T.L.M. Spatiotemporal endothelial cell–pericyte association in tumors as shown by high resolution 4d intravital imaging. Sci. Rep. 2018, 8, 9596.
- Boulch, M.; Grandjean, C.L.; Cazaux, M.; Bousso, P. Tumor immunosurveillance and immunotherapies: A fresh look from intravital imaging. Trends Immunol. 2019, 40, 1022–1034.
- Nezu, A.; Morita, T.; Nagai, T.; Tanimura, A. Simultaneous monitoring of ca(2+) responses and salivary secretion in live animals reveals a threshold intracellular ca(2+) concentration for salivation. Exp. Physiol. 2019, 104, 61–69.
- Tvrdik, P.; Kearns, K.N.; Sharifi, K.A.; Sluzewski, M.F.; Acton, S.T.; Kalani, M.Y.S. Calcium imaging of microglial network activity in stroke. In Microglia: Methods and Protocols; Garaschuk, O., Verkhratsky, A., Eds.; Springer New York: New York, NY, USA, 2019; pp. 267–279.
- Archambault, L.S.; Trzilova, D.; Gonia, S.; Gale, C.; Wheeler, R.T.; Gacser, A.; Hube, B. Intravital imaging reveals divergent cytokine and cellular immune responses to candida albicans and candida parapsilosis. mBio 2019, 10, e00266-19.
- Shandala, T.; Lim, C.; Sorvina, A.; Brooks, D.A. A drosophila model to image phagosome maturation. Cells 2013, 2, 188–201.
- Koyama, L.A.J.; Aranda-Díaz, A.; Su, Y.H.; Balachandra, S.; Martin, J.L.; Ludington, W.B.; Huang, K.C.; O’Brien, L.E. Bellymount enables longitudinal, intravital imaging of abdominal organs and the gut microbiota in adult drosophila. PLoS Biol. 2020, 18, e3000567.
- Sorvina, A.; Brooks, D.A.; Ng, Y.S.; Bader, C.A.; Weigert, R.; Shandala, T. Bacterial challenge initiates endosome-lysosome response in drosophila immune tissues. IntraVital 2013, 2, e23889.
- Asokan, N.; Daetwyler, S.; Bernas, S.N.; Schmied, C.; Vogler, S.; Lambert, K.; Wobus, M.; Wermke, M.; Kempermann, G.; Huisken, J.; et al. Long-term in vivo imaging reveals tumor-specific dissemination and captures host tumor interaction in zebrafish xenografts. Sci. Rep. 2020, 10, 13254.
- Revskoy, S.; Blair, M.E.; Powell, S.M.; Hausman, E.S.; Blackburn, J.S. In vivo imaging defines vascular interplay in the development of lymphocytic leukemia in zebrafish models. bioRxiv 2019, 806562.
- Sedin, J.; Giraud, A.; Steiner, S.E.; Ahl, D.; Persson, A.E.G.; Melican, K.; Richter-Dahlfors, A.; Phillipson, M. High resolution intravital imaging of the renal immune response to injury and infection in mice. Front. Immunol. 2019, 10, 2744.
- Upadhaya, S.; Krichevsky, O.; Akhmetzyanova, I.; Sawai, C.M.; Fooksman, D.R.; Reizis, B. Intravital imaging reveals motility of adult hematopoietic stem cells in the bone marrow niche. Cell Stem Cell 2020, 27, 336–345.e334.
- Lai, C.W.; Chen, H.L.; Yen, C.C.; Wang, J.L.; Yang, S.H.; Chen, C.M. Using dual fluorescence reporting genes to establish an in vivo imaging model of orthotopic lung adenocarcinoma in mice. Mol. Imaging Biol. 2016, 18, 849–859.
- Ikeda, W.; Sasai, K.; Akagi, T. Imaging window device for subcutaneous implantation tumor. Methods Mol. Biol. (Clifton N. J.) 2018, 1763, 153–163.
- Ryu, C.M.; Yu, H.Y.; Lee, H.Y.; Shin, J.H.; Lee, S.; Ju, H.; Paulson, B.; Lee, S.; Kim, S.; Lim, J.; et al. Longitudinal intravital imaging of transplanted mesenchymal stem cells elucidates their functional integration and therapeutic potency in an animal model of interstitial cystitis/bladder pain syndrome. Theranostics 2018, 8, 5610–5624.
- Fumagalli, A.; Bruens, L.; Scheele, C.; van Rheenen, J. Capturing stem cell behavior using intravital and live cell microscopy. Cold Spring Harb. Perspect. Biol. 2020, 12, a035949.
- Balan, M.; Trusohamn, M.; Ning, F.C.; Jacob, S.; Pietras, K.; Eriksson, U.; Berggren, P.-O.; Nyqvist, D. Noninvasive intravital high-resolution imaging of pancreatic neuroendocrine tumours. Sci. Rep. 2019, 9, 14636.
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239.
- Kremers, G.J.; Gilbert, S.G.; Cranfill, P.J.; Davidson, M.W.; Piston, D.W. Fluorescent proteins at a glance. J. Cell Sci. 2011, 124, 157–160.
- Chudakov, D.M.; Matz, M.V.; Lukyanov, S.; Lukyanov, K.A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 2010, 90, 1103–1163.
- Shaner, N.C.; Steinbach, P.A.; Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2005, 2, 905–909.
- Megason, S.G.; Fraser, S.E. Imaging in systems biology. Cell 2007, 130, 784–795.
- Oliva Trejo, J.A.; Tanida, I.; Suzuki, C.; Kakuta, S.; Tada, N.; Uchiyama, Y. Characterization of starvation-induced autophagy in cerebellar purkinje cells of phluorin-mkate2-human lc3b transgenic mice. Sci. Rep. 2020, 10, 9643.
- Bozhanova, N.G.; Baranov, M.S.; Klementieva, N.V.; Sarkisyan, K.S.; Gavrikov, A.S.; Yampolsky, I.V.; Zagaynova, E.V.; Lukyanov, S.A.; Lukyanov, K.A.; Mishin, A.S. Protein labeling for live cell fluorescence microscopy with a highly photostable renewable signal. Chem. Sci. 2017, 8, 7138–7142.
- Weissman, T.A.; Pan, Y.A. Brainbow: New resources and emerging biological applications for multicolor genetic labeling and analysis. Genetics 2015, 199, 293–306.
- Roo, J.J.d.; Vloemans, S.A.; Vrolijk, H.; Haas, E.F.d.; Staal, F.J. Development of an in vivo model to study clonal lineage relationships in hematopoietic cells using brainbow2.1/confetti mice. Future Sci. OA 2019, 5, FSO427.
- El-Nachef, D.; Shi, K.; Beussman, K.M.; Martinez, R.; Regier, M.C.; Everett, G.W.; Murry, C.E.; Stevens, K.R.; Young, J.E.; Sniadecki, N.J.; et al. A rainbow reporter tracks single cells and reveals heterogeneous cellular dynamics among pluripotent stem cells and their differentiated derivatives. Stem Cell Rep. 2020, 15, 226–241.
- Cook, Z.T.; Brockway, N.L.; Tobias, Z.J.C.; Pajarla, J.; Boardman, I.S.; Ippolito, H.; Nkoula, S.N.; Weissman, T.A. Combining near-infrared fluorescence with brainbow to visualize expression of specific genes within a multicolor context. Mol. Biol. Cell 2019, 30, 491–505.
- Specht, E.A.; Braselmann, E.; Palmer, A.E. A critical and comparative review of fluorescent tools for live-cell imaging. Annu. Rev. Physiol. 2017, 79, 93–117.
- Wei, L.; Chen, Z.; Shi, L.; Long, R.; Anzalone, A.V.; Zhang, L.; Hu, F.; Yuste, R.; Cornish, V.W.; Min, W. Super-multiplex vibrational imaging. Nature 2017, 544, 465–470.
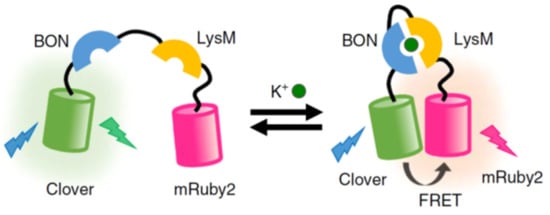
- Shen, Y.; Wu, S.-Y.; Rancic, V.; Aggarwal, A.; Qian, Y.; Miyashita, S.-I.; Ballanyi, K.; Campbell, R.E.; Dong, M. Genetically encoded fluorescent indicators for imaging intracellular potassium ion concentration. Commun. Biol. 2019, 2, 18.
- Udensi, U.K.; Tchounwou, P.B. Potassium homeostasis, oxidative stress, and human disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111–122.
- Yong, K.-T.; Law, W.-C.; Hu, R.; Ye, L.; Liu, L.; Swihart, M.T.; Prasad, P.N. Nanotoxicity assessment of quantum dots: From cellular to primate studies. Chem. Soc. Rev. 2013, 42, 1236–1250.
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819.
- Goreham, R.V.; Schroeder, K.L.; Holmes, A.; Bradley, S.J.; Nann, T. Demonstration of the lack of cytotoxicity of unmodified and folic acid modified graphene oxide quantum dots, and their application to fluorescence lifetime imaging of hacat cells. Microchim. Acta 2018, 185, 128.
- Mei, K.-C.; Rubio, N.; Costa, P.M.; Kafa, H.; Abbate, V.; Festy, F.; Bansal, S.S.; Hider, R.C.; Al-Jamal, K.T. Synthesis of double-clickable functionalised graphene oxide for biological applications. Chem. Commun. 2015, 51, 14981–14984.
- Jiang, D.; Chen, Y.; Li, N.; Li, W.; Wang, Z.; Zhu, J.; Zhang, H.; Liu, B.; Xu, S. Synthesis of luminescent graphene quantum dots with high quantum yield and their toxicity study. PLoS ONE 2016, 10, e0144906.
- Mitchell, B.; Bradley, S.J.; Thomas, N. Graphene quantum dots. Part. Part. Syst. Charact. 2014, 31, 415–428.
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699.
- Chung, S.; Revia, R.A.; Zhang, M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv. Mater. 2021, 33, 1904362.
- Sweetman, M.J.; Hickey, S.M.; Brooks, D.A.; Hayball, J.D.; Plush, S.E. A practical guide to prepare and synthetically modify graphene quantum dots. Adv. Funct. Mater. 2019, 29, 1808740–1808758.
- Wang, X.; Wang, Y.; He, H.; Chen, X.; Sun, X.; Sun, Y.; Zhou, G.; Xu, H.; Huang, F. Steering graphene quantum dots in living cells: Lighting up the nucleolus. J. Mater. Chem. B 2016, 4, 779–784.
- Zhao, Q.; Huang, C.; Li, F. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524.
- Suhling, K.; French, P.M.W.; Phillips, D. Time-resolved fluorescence microscopy. Photochem. Photobiol. Sci. 2005, 4, 13–22.
- Gillam, T.A.; Sweetman, M.J.; Bader, C.A.; Morrison, J.L.; Hayball, J.D.; Brooks, D.A.; Plush, S.E. Bright lights down under: Metal ion complexes turning the spotlight on metabolic processes at the cellular level. Coord. Chem. Rev. 2018, 375, 234–255.
- Lavis, L.D.; Raines, R.T. Bright ideas for chemical biology. ACS Chem. Biol. 2008, 3, 142–155.
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123.
- Miller, D.R.; Jarrett, J.W.; Hassan, A.M.; Dunn, A.K. Deep tissue imaging with multiphoton fluorescence microscopy. Curr. Opin. Biomed. Eng. 2017, 4, 32–39.
- Zheng, Q.; Juette, M.F.; Jockusch, S.; Wasserman, M.R.; Zhou, Z.; Altman, R.B.; Blanchard, S.C. Ultra-stable organic fluorophores for single-molecule research. Chem. Soc. Rev. 2014, 43, 1044–1056.
- Lei, Z.; Li, X.; Luo, X.; He, H.; Zheng, J.; Qian, X.; Yang, Y. Bright, stable, and biocompatible organic fluorophores absorbing/emitting in the deep near-infrared spectral region. Angew. Chem. Int. Ed. 2017, 56, 2979–2983.
