Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vishwanath Managuli and Version 2 by Catherine Yang.
Nosocomial infections, termed hospital-acquired infections (HAIs), are acquired from a healthcare or hospital setting. HAI is mainly caused by bacteria, such as Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, Enterococci spp., Methicillin-resistant Staphylococcus aureus (MRSA), and many more. Due to growing antibacterial resistance, nanotechnology has paved the way for more potent and sensitive methods of detecting and treating bacterial infections.
- hospital-acquired infections
- nanotechnology
- Nosocomial Infections
- Healthcare
- Multi-Drug Resistance
1. Introduction
The term ‘nosocomial’ is derived from two Greek words, ‘nosus’ and ‘komeion’ that literally translate into ‘disease’, and ‘take care of’. It was during the first half of the 18th century that the scientific study of nosocomial infection or hospital-acquired infection (HAI) started [1]. During the post-World War II era, various antibiotics were discovered and widely used in treatment. Penicillin was used extensively, which caused the death rate due to postoperative pneumonia to reduce from 30% to <10% and surgical wound infections to reduce below 5%. By the 1960s, the surgical infection rate reduced below 2% due to the introduction of other antibiotics [2]. However, this soon led to a major penicillin-resistant Staphylococcal epidemic amongst patients and health care workers and many of them were nasal and dermal carriers [3]. After the discovery of methicillin and vancomycin in the 1960s, the emergence of methicillin-resistant Staphylococcus aureus (MRSA) began due to overuse. Along with MRSA, other resistant organisms that emerged were vancomycin-resistant Enterococcus spp., a few members of the Enterobacteriaceae family, Pseudomonas aeruginosa, Streptococcus spp., and Candida spp. Hence, nosocomial infections are infections that occur in a patient while receiving care in a hospital or other health care facility [4]. After an infection is confirmed to be of nosocomial origin, according to the definition mentioned before, the specific type of infection is categorized based on the systemic classification provided by the National Health Safety Network (NHSN) with the Centers for Disease Control and Prevention (CDC) which are specifically based on clinical and biological criteria [5]. According to the NHSN and CDC criteria, HAIs have been classified into 14 different types. Out of these, the incidences of device associated HAIs (DA-HAI) are the most common in healthcare settings which include central-line associated bloodstream infections (CLABSI), catheter-related bloodstream infections (CRBSI), catheter-associated urinary tract infections (CAUTI), ventilator-associated pneumonia (VAP), and surgical site infections (SSI) [6]. The most common nosocomial infection-causing bacteria include S. aureus, including antibiotic-resistant MRSA, Escherichia coli, Enterococcus spp., and Candida spp. With the increase in antibiotic resistance of nosocomial infections, nanoparticles, especially metallic nanoparticles, provide a successful alternative due to their special properties including high reactivity and stability. Nanoparticles increase the permeability of bacterial cell membranes, resulting in a higher uptake of antibiotics by the bacterial cells [7].
2. Nano Strategies Combating Nosocomial Infections
The development of antibiotic resistance is a rising crisis today. Further, genetic tolerance against antibiotics in bacteria such as MRSA is a common occurrence. Nanomaterials inhibit bacterial growth or activity that results in infections. Nanoparticles penetrate the bacteria and biofilm leading to reactive oxygen species (ROS) generation that eliminates bacteria. Thus, nanoparticles are a novel approach to combat drug-resistant bacterial infections. Different nanomaterials, such as nanoparticles and nanotubes, are directly used in biomedical devices to prevent spreading infections. Owing to the small size and high surface area to volume ratio, nanoparticles (NPs) have a much stronger physical interaction capability with bacterial cells compared to microparticles. These NPs offer many interaction mechanisms between nanomaterials and cell walls, such as changing the membrane permeability by penetration, blocking oxidative phosphorylation, or by generating free radicals leading to damage of the cell membrane, and in turn cell death, thus increasing the oxidative stress and destroying DNA [8][52], as shown in Figure 12. Additionally, the ionic activity of nanoparticles can modulate the bacterial signal transduction leading to the inhibition of bacterial growth or inactivating the enzymes by interacting with them. There are physical interaction mechanisms too, which include bacterial wrapping to induce surface stresses and penetration through sharp edges that causes physical damage and adverse chemical effects [9][10][53,54]. Similarly, helpful is the creation of anti-adhesion surfaces that inhibit biofilm formation [11][55].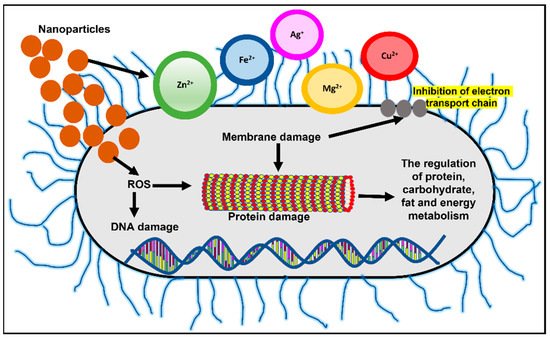
2.1. Nanoparticles Based Molecular Beacons
Nanoparticles can be used as molecular beacons to identify relevant bacterial strains in minimal time [13][14][15][16][17][18][57,58,59,60,61,62]. This is especially important in emergency cases or in an ICU where immediate results are required to continue medical procedures. Molecular beacons are the most promising method for qualitative and quantitative biological detection of bacteria. In a recent study, molecular beacons loaded with nanoparticles have been used for the rapid detection of bacteria and viruses (Figure 23a). In another recent study, it was observed that hybridizing molecular beacons with nanoparticles improved the bacterial efficiency. The new age nanoprobes comprised of molecular beacons hybridized to gold nanoparticles could detect E. coli at a concentration of 102 cfu/mL. This method was 1000 times more sensitive than detecting using only molecular beacons [13][57].
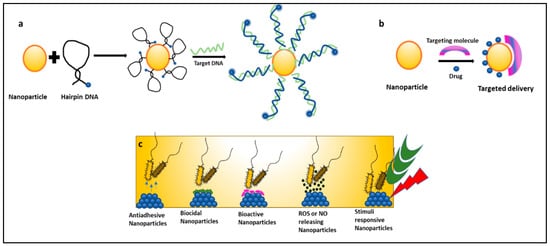

Figure 23. Mechanisms of nanoparticles combating nosocomial infections: (a) nanoparticles as molecular beacons, (b) nanoparticles for targeted drug delivery of antibiotics, and (c) types of nanoparticles preventing biofilm-associated nosocomial infections.
Various NPs are employed in life science to accurately detect a bacterial strain using the following techniques: Surface-enhanced Raman Spectroscopy (SERS) detection method, the rapid and cost-effective immuno-magnetic separation technique, and short detection time-based fluorescence microscopy, as well as fast and visual methods like the calorimetric method or highly sensitive methods like real-time PCR techniques [8][14][15][52,58,59]. In sandwich-structure immunoassays, E. coli and S. aureus were detected using the SERS method in a total assay time of 10 min [16][60]. Similarly, in another study, E. coli and S. epidermidis were detected using the SERS method in 10 min by employing the synthesis of AgNPs coating on the cell wall of bacteria [17][61]. This leads to the 30 times increase in the Raman signal of these bacteria compared to that obtained using a mixing of colloid and bacterial suspension. Additionally, reports are showing the presence of cost-friendly techniques to detect bloodstream infection using magneto fluorescent NPs which proved to be time-saving and equally sensitive methods [18][62]. Apart from this, the inherent superiority of molecular beacon probes and biofunctionalized NPs led to a series of novel principles, methods, and techniques to exploit bioanalytical and biomedical studies as well.
2.2. Nanoparticles Formulated with Drugs and Antibiotics-Nano Bactericidal
Nanomedicine is an offshoot of nanotechnology under exploration for its suitability in the delivery of treatment and healthcare benefits. It provides platforms like targeted drug delivery using different nanoparticulate systems, a multidrug complex entrapped in a single nanoparticle, surface functionalization with nano-particulate antibiotic materials, nano antibiotics, and creating ROS using inorganic metal oxide NPs, playing important roles in the restoration of antibiotic activity (Figure 23c) [19][20][63,64]. Antibiotics conjugated with NPs give the advantage of lower sample consumption and higher sensitivity [21][65]. Studies have reported that nanoparticles coupled with antibiotics, such as vancomycin, amoxicillin, and penicillin G, show an enhanced antimicrobial resistance against S. aureus and E. coli [22][66]. NO (Nitric oxide) releasing nanoparticles and metal oxide nanoparticles (TiO2, ZnO) have also shown effective antimicrobial activity against many MDR strains and are under exploration [8][52]. In a recent study, gold nanoparticles (Au NPs) were conjugated with ceragenin CSA-131 (cationic steroid antimicrobial) and were tested for their bactericidal activity against S. aureus, S. epidermidis, K. pneumoniae, K. oxytoca, and P. aeruginosa. All nanosystems exhibited potent bactericidal activity by generating ROS, resulting in the damage of the bacterial membranes and the leakage of intracellular content [23][67]. Researchers have taken an interest in exploring the suitability of nano-photo thermal therapy for MDR bacteria. This therapy involves the selective killing of bacteria by the transfer of heat generated from the conversion of electromagnetic radiation. It irreversibly damages the bacterial membranes and interferes with cell wall biosynthesis. These outcomes are a boon to researchers as antibiotics are turning out to be inefficient [23][24][67,68].
2.3. Nanotechnology in the Development of Drug Delivery Systems (Nano-DDS)
Drugs with poor solubility and absorption ability can be delivered via nanoparticles for target-specific drug delivery. However, the efficacy of these nanoparticles for drug delivery depends on factors such as size, shape, and other physical/chemical characteristics [25][20]. Malachite green (MG) encapsulated in mesoporous silica nanoparticles (MSN) was tested against common nosocomial infection-causing bacteria such as S. aureus and E. coli. MG-MSN was found to be effective against both tested bacterial strains. S. aureus was more phototoxic to MG-MSN compared to E. coli. The anti-biofilm efficacy of MG-MSN on E. coli and S. aureus were also studied. Biofilm inhibition was found to be 65.68 ± 2.62% in E. coli and 79.66 ± 3.82% in S. aureus [26][69]. Nano-DDS is a promising approach in the control of HCAI, but only a small number of nano-DDS products were commercially successful in the market [27][28][70,71]. This is mostly due to financial profitability and poor information about product functioning available to health professionals across countries, leading to further delay in its full exploitation concerning generic medicine. Similarly, concerns about the toxicity of nanoparticles, their effect on the blood-brain barrier, and their delivery to the central nervous system are limiting their application in medicine. Industrial production, quality control, and storage stability of nanoparticles must be assessed to ensure their purity and safe administration in a biological system [29][72]. Nevertheless, the progress of the research in this domain promises a higher success ratio in defending the patient’s interests.
2.4. Surface Modifications to Control Biofilm-Associated Infections
Biofilm-based infections are important causes of morbidity, affecting millions of people every year, typically causing chronic nosocomial infections. The current clinical practice with biofilm-associated infection is to treat it with high-dose antibiotics and if symptoms persist, a surgical replacement can be done to reduce further complications to patient health [30][73]. Tailoring the functional surface properties of implants or biomedical devices used during treatments can curb the initial and later stages of infection development. Nanoparticles facilitate the sustained release of attached bioactive materials or ions and thus provide longer antibiofilm activity. In a study conducted on the antibacterial activity of silver nanoparticles (AgNPs) against nosocomial A. baumannii AIIMS 7 in biofilm mode, nanoparticles exhibited significant biofilm disruption activity at a minimum inhibitory concentration of 2 mg/mL. The eradication of the biofilm was improved on combined exposure to AgNPs and antibiotics. These nanoparticles inhibited bacterial growth through intracellular oxidative stress and interact with thiol-groups in cellular proteins resulting in denaturation [31][74]. Similarly, surface modification of other nanoparticles such as AuNPs makes them desirable to be used for oral biology [32][75] and other healthcare applications. A detailed study on various types of nanoparticles and their results are tabulated in Table 1.
Methodologies like anti-fouling, anti-adhesive or bactericidal coating, and many more methods are adopted for reducing bacterial adhesion on medical devices [30][33][73,76]. These multifunctional coatings simultaneously promote osseointegration and prevent infection of implants. Silver is the most common bactericidal agent used to date due to its broad spectrum of antimicrobial activity against both Gram-positive and negative bacterial strains [33][76]. The inorganic nanoparticles of Ag and Au were used in coating urethral [34][77], venous and ventricular catheters [35][78], organic nanoparticles based on chitosan and PEG stabilized lipid in bone and dental implants [36][79], as well as several other metallic/metal-polymer composites in the development of face masks, heart valves, pedicle screws, contact lenses, and orthopedic and oral implants depending on a working mechanism [37][80].
To address the looming threat of nosocomial infection spread, there is an urgent need for a comprehensive action plan at the national and international levels. An independent review by a committee sponsored and supported by Wellcome Trust and the Department of Health, UK provides a deeper insight and solutions. HAI account for 0.7 million deaths globally, causes the death of nearly 60,000 newborns in India each year, two million infections in the USA alone, and caused 20 billion USD in excess costs. Additionally, the death toll may increase to 10 million by 2050. Therefore, the committee came up with a suggestion of tackling antimicrobial resistance on ten different fronts [38][93]. The list includes Public Awareness, Surveillance, Human Capital, Vaccines and Alternatives, a Global Innovation Fund, Rapid Diagnostics kits to guide doctors, and an International Coalition for action, etc. In this way, nanotechnology can play a major role and offer solutions in rapid diagnostics, control of the spread, and vaccine effectiveness.
