Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Amina Yu and Version 2 by Amina Yu.
IAsthma is no lon all asthma patients, the diseaseger considered a single disease entity but rather an umbrella term used to describe a collection of various phenotypes and endotypes. Asthma manifests as an obstruction of airflow due to airway hyper-responsiveness, leading to symptoms of wheezing, shortness of breath, cough, and chest tightness. Revelations in pathophysiology of asthma have uncovered underlying heterogeneity, and “asthma” is no longer considered a single disease entity but rather an umbrella term used to describe a collection of various phenotypes and endotypes.
- asthma
- COVID-19
- biologics
- omalizumab
1. Asthma, COVID-19, and ACE2 Interrelationship
A possible protective effect of a Th2-high asthma endotype against poor clinical outcomes of COVID-19 has been suggested (Figure 1). It was showed that among patients with asthma, those with allergic asthma were at a lower risk of COVID-19 morbidity and mortality than patients with non-allergic asthma [1]. According to experimental studies, eosinophils may have a role in promoting respiratory virus clearance and antiviral host defense, which leads to the postulation that asthma patients with type 2 inflammation characterized by an increased number of eosinophils in the airway might be protected against severe COVID-19 outcomes [2][3]. In a retrospective cohort study conducted in Wuhan, China including 59 patients with confirmed COVID-19 and underlying chronic respiratory disease (chronic bronchitis, chronic obstructive pulmonary disease, or asthma), 73% of patients suffering severe COVID-19 had a low blood eosinophil count of less than 0.2 × 109 cells/L compared to 24% of patients who had non-severe COVID-19 (p < 0.001) [4]. In a retrospective study including 951 patients with asthma and COVID-19 conducted in the United States, pre-existing eosinophilia defined as a blood eosinophil count of ≥150 cells/µL was associated with reduced COVID-19-related hospitalization and mortality [5]. In the same study, among patients who had eosinopenia (absolute eosinophil count of 0 cells/µL) at the time of hospitalization, those in whom the eosinophil count increased to above ≥150 cells/µL during admission were significantly less likely to die (mortality rate 9.6%) compared to patients whose eosinophil count remained <150 cells/µL throughout hospitalization (mortality rate 25.8%) (odds ratio [OR], 0.006; 95% confidence interval [CI], 0.0001–0.64; p = 0.03). In contrast, Th2-low endotype of asthma is characterized by neutrophilia, which is associated with a neutrophil extracellular trap formation and promotion of tissue injury, as well as an increased level of IL-17, which results in the propagation of proinflammatory cytokines [6].
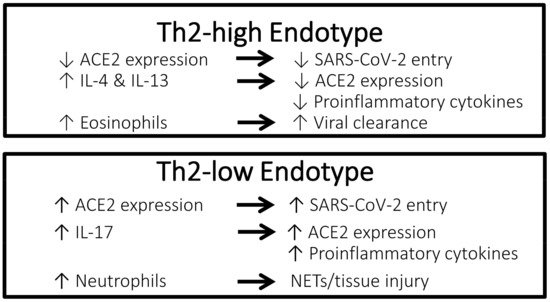
Figure 1. Characteristics of the Th2-high endotype asthma vs. Th2-low endotype asthma that may confer different effects against COVID-19. ACE2, angiotensin-converting enzyme 2 receptor; COVID-19, coronavirus disease 2019; IL, interleukin; NETs, neutrophil extracellular traps; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Th2, T-helper type 2.
Rhinoviruses are picoronaviruses that gain entrance into host airway epithelial cells via intracellular adhesion molecule 1 (ICAM-1), a low-density lipoprotein receptor (LDLR), and cadherin-related family member 3 (CDHR3) [7][8]. In patients with chronic allergic asthma, epithelial barrier disruption may lead to increased accessibility of rhinoviruses to CDHR3, which is mainly localized on cell surfaces along intercellular junctions [8]. In contrast, angiotensin-converting enzyme-2 (ACE2) has been identified as the receptor for the SARS-CoV-2 spike protein that provides the entryway for the virus into host cells [9]. Studies have reported a correlation between increased ACE2 expression and increased infectivity of SARS-CoV-2 [10][11]. Compared to airway cells of patients with non-allergic asthma, cells of patients with allergic asthma show lower ACE2 expression [12]. Exposure to the type 2 cytokines IL-4 and IL-13 was shown to reduce ACE2 expression in airway epithelial cells [13]. Furthermore, using a blood eosinophil count as a biomarker for type 2 inflammation, cut-off values of 150 and 300 cells/µL effectively identified patients with differential expression levels of ACE2 [14]. Therefore, evidence suggests that type 2 inflammation characteristic of the Th2-high asthma endotype is associated with lower ACE2 expression in the airway, thus potentially conferring a protective effect against COVID-19. Conversely, ACE2 expression has been shown to be upregulated by IL-17, which is elevated in the Th2-low asthma endotype [15].
2. Role of Airway Epithelium in COVID-19 and Asthma
Previously, airway epithelium was considered to simply serve as a mechanical barrier enabling gas exchange. It is now understood that airway epithelium is a complex tissue that performs a multitude of crucial functions, including the mediation of immune mechanisms [16]. The sinonasal airway epithelium is the initial site of SARS-CoV-2 infection and viral replication, and at this stage of infection, ciliated and mucus-secreting goblet cells that express ACE2 are the primary targets [17]. Early stages of COVID-19 are typically associated with relatively mild symptoms, likely related to the dampening of interferon-driven innate immune response to SARS-CoV-2 in nasal and bronchial epithelium [18][19]. Following the initial stage, the disease extends down the respiratory tract to the gas exchange portion of the lung where ACE2-expressing alveolar type II cells become the primary target of viral entry and replication [17]. Whereas in the nasal epithelium, damaged ciliated and secretory cells are replaced by progenitor basal cells that are spared from viral destruction [20], damage to alveolar type II cells results in much more dire consequences. Not only do alveolar type II cells secrete functional surfactant, they are also the progenitor cells for epithelial cells, and their destruction leads to gas exchange dysfunction, alveolar flooding from disrupted epithelium, and initiation of an innate immune response which further propagates alveolar damage due to inflammation [17].
In the context of asthma, the coordinated protective mechanisms of airway epithelium are disturbed differently in Th2-high (type 2 inflammation) and Th2-low (non-type 2 inflammation) asthma endotypes/phenotypes. The dysfunction of ciliated and secretory cells is observed in both asthma types; however, in Th2-high asthma the key early activators released by epithelial cells in response to allergens are IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), whereas in Th2-low asthma, TNF-α, IL-6, IL-8, and IL-1β are the main inflammatory mediators released in response to environmental factors [21]. Importantly, in both asthma types, viruses are among the external factors that can instigate asthma pathogenesis at the epithelium, likely in part due to epigenetic mechanisms by which gene expression is modulated by DNA methylation, histone modulation, or translation modification by microRNAs in response to external stimuli [16][22]. How all of these factors are influenced by COVID-19 requires further study.
3. Therapeutic Management of Asthma Patients during the COVID-19 Pandemic
As asthma has not been clearly shown to be a significant risk factor for SARS-CoV-2 infection or poor outcomes in COVID-19, major modification to established guideline-recommended standard asthma treatment during the COVID-19 pandemic has not been deemed necessary. According to the Global Initiative for Asthma (GINA) guidance about COVID-19 and asthma updated in March 2021, it is important to continue good asthma management in order to maintain optimal symptom control, to reduce the risk of severe exacerbations, and to minimize the need for oral corticosteroids, thus reducing the need to seek urgent medical care and consequential potential exposure to SARS-CoV-2 [23]. In a nationwide health insurance claims data-based study conducted in Korea, which included 218 patients with confirmed COVID-19 and underlying asthma, in univariate analyses, use of short-acting beta agonists was a significant risk factor for intensive care unit admission and use of long-acting beta agonists was a significant protective factor for hospital admission duration, though these factors were no longer significant in multivariate analyses [24]. In terms of the total medical cost burden associated with COVID-19, use of oral short-acting beta agonists in the past year was an independent risk factor for increased cost burden. Furthermore, compared to patients with GINA step 1 asthma (mild asthma not requiring maintenance treatment), patients with GINA step 5 asthma (moderate-to-severe asthma that is difficult to control despite a medium/high dose inhaled corticosteroid plus long-acting beta agonist) required a longer duration of hospitalization for COVID-19 in both univariate and multivariate analyses. These findings support that optimal asthma control with an adequate use of maintenance therapies may improve prognosis in patients with asthma who contract COVID-19; therefore, patients should be advised to continue taking their prescribed asthma medications, including biologic therapy and inhaled or oral corticosteroids, as is recommended by GINA. In addition, all patients should have a written asthma action plan that advises on controller and reliever medication use in case of worsening asthma symptoms. In addition, GINA guidance recommends the avoidance of nebulizer use as a precaution against virus transmission via airborne particles, and that switching to pressurized metered dose inhalers (with a spacer if needed) is preferable. Similarly, avoidance of spirometry during healthcare visits is also recommended as a transmission-based precaution.
4. COVID-19 Vaccination in Patients with Asthma
In addition to maintaining optimal symptom control by continuing all prescribed asthma medications during the pandemic, patients with asthma should be further protected with COVID-19 vaccination. Patients with chronic allergic and atopic diseases, including those treated with biologic agents, have not exhibited increased risk for hypersensitivity reactions after a COVID-19 vaccination [25]. Based on presently understood risks and benefits, GINA guidance recommends that people with asthma undergo COVID-19 vaccination, and the Pfizer/BioNTech and Moderna COVID-19 vaccines were specifically mentioned in the GINA guidance document released in October 2021 [23]. The safety and tolerability of the mRNA COVID-19 Pfizer/BioNTech vaccine in 253 patients with severe asthma were evaluated in a survey study conducted in Italy [26]. According to patient-reported results collected via a vaccination-related adverse events questionnaire, over 80% of patients did not report adverse events after receiving the first and second doses of the vaccine. Among patients who did report experiencing adverse events following vaccination, the reported effects were mostly very common effects such as injection site pain and swelling, weakness, fever, myalgia, arthralgia, and headache, reported by 80% of patients after the first dose and 95% after the second dose, and no patient reported experiencing a rare post-vaccination side effect, including facial asymmetry or severe allergic reaction. In this population of severe asthma patients, 220 (87%) patients were receiving ongoing biologic treatment, and proportions of patients experiencing adverse events following the first and second vaccine doses were comparable across biologic agents (benralizumab 16.9–23.1%, mepolizumab 19.3–20.7%, omalizumab 21.8–22.8%, dupilumab 11.1%). According to GINA guidance, patients with asthma should continue to receive the annual influenza vaccine, with a separation of at least 14 days between COVID-19 and influenza vaccinations. Finally, guidance suggests that biologic therapy and a COVID-19 vaccine should not be given on the same day to allow adverse effects of either to be more easily distinguishable.
5. Impact of Oral and Inhaled Corticosteroids on COVID-19
Compared to patients with asthma of Th2-low endotype, those with Th2-high endotype show higher responsiveness to corticosteroid treatment [27]. As corticosteroids have immunosuppressive effects, the impact of corticosteroids on COVID-19 outcomes may be concerning for many clinicians [28]. Previous studies of corticosteroid treatment during respiratory viral illness, including SARS, have generally shown a lack of effectiveness in reducing morbidity and instead, possible harm [29][30]. Earlier in the pandemic, when COVID-19-specific evidence relating to corticosteroid use was still largely lacking, the World Health Organization (WHO) initially recommended against the use of systemic corticosteroids to treat COVID-19 in its clinical management guidance document released in March 2020 [31]. However, as studies evaluating various treatments, including corticosteroids, in patients with COVID-19 have rapidly accumulated, the WHO changed its stance in a guidance document released in September 2020, which stated a strong recommendation for the use of systemic corticosteroids in the treatment of patients with severe and critical COVID-19 based on the most current evidence [32]. The updated WHO guidance specifically referenced the preliminary report of the now published RECOVERY multicenter, randomized controlled trial, which included 6425 hospitalized patients with COVID-19 who were randomized to receive oral or intravenous dexamethasone 6 mg once daily in addition to usual care or usual care alone [33]. Final results of the RECOVERY study showed that dexamethasone-treated COVID-19 patients had a significantly lower risk of the primary outcome of 28-day mortality compared to those who received usual care alone (age-adjusted rate ratio, 0.83; 95% CI, 0.75–0.93; p < 0.001) [33]. However, in the subgroup analysis according to respiratory support at randomization, mortality risk was significantly reduced in patients requiring invasive mechanical ventilation or oxygen only, but was not significantly reduced in patients who did not require respiratory support. In the PRINCIPLE study [34], which is a randomized controlled trial, older non-hospitalized patients with COVID-19 (65 years of older or 50 years or older with comorbidities) who received inhaled budesonide 800 µg twice daily for 14 days in addition to usual care had a significantly shorter time to recovery by approximately 3 days compared to those who received usual care alone.
The burden of COVID-19 among patients with chronic respiratory diseases has not been shown to be increased, and indeed was shown to be decreased in some studies, compared to the general population [35]. This rather unexpected finding has been hypothesized to be attributed to potential protective effects of respiratory disease treatments against SARS-CoV-2 infection and severe disease. Observational studies have shown that inhaled corticosteroid use in patients with asthma was associated with the increased prevalence of non-COVID-19 upper and lower respiratory tract infections [36][37]. In contrast, inhaled corticosteroid treatment has been associated with a reduced expression of ACE2, the entryway of SARS-CoV-2 into host cells, thereby potentially reducing SARS-CoV-2 susceptibility and morbidity [38][39]. Results of an in vitro study have shown that pre-treatment of human nasal and tracheal epithelial cells with budesonide, glycopyrronium, and formoterol inhibited coronavirus HCoV-229E replication and cytokine production [40]. An in vitro study has revealed that the corticosteroids mometasone and ciclesonide suppressed replication of SARS-CoV-2 in a culture medium of infected cells, and that ciclesonide was particularly effective in a concentration-dependent manner [41]. Furthermore, a study that screened a panel of 48 United States Food and Drug Administration-approved drugs identified ciclesonide as an inhibitor of SARS-CoV-2 cytopathic viral activity [42]. In addition to the inhibitory effects on viral replication, treatment with corticosteroids might also reduce the severity of COVID-19 by inhibiting virus-induced cytokine release and dampening the exaggerated inflammatory response responsible for severe symptoms [43]. Overall, the effects of the appropriate use of oral and inhaled corticosteroids is expected to lean toward being beneficial rather than harmful for patients with asthma during the pandemic, and adherence to all maintenance asthma medications to ensure good symptom control and to prevent exacerbation should be emphasized, as is consistently recommended by professional organizations worldwide [44].
References
- Yang, J.M.; Koh, H.Y.; Moon, S.Y.; Yoo, I.K.; Ha, E.K.; You, S.; Kim, S.Y.; Yon, D.K.; Lee, S.W. Allergic disorders and susceptibility to and severity of COVID-19: A nationwide cohort study. J. Allergy Clin. Immunol. 2020, 146, 790–798.
- Carli, G.; Cecchi, L.; Stebbing, J.; Parronchi, P.; Farsi, A. Is asthma protective against COVID-19? Allergy 2021, 76, 866–868.
- Rosenberg, H.F.; Dyer, K.D.; Domachowske, J.B. Respiratory viruses and eosinophils: Exploring the connections. Antiviral Res. 2009, 83, 1–9.
- Chen, D.; Zhang, S.; Feng, Y.; Wu, W.; Chang, C.; Chen, S.; Zhen, G.; Yi, L. Decreased eosinophil counts and elevated lactate dehydrogenase predict severe COVID-19 in patients with underlying chronic airway diseases. Postgrad. Med. J. 2021, 1–8.
- Ferastraoaru, D.; Hudes, G.; Jerschow, E.; Jariwala, S.; Karagic, M.; de Vos, G.; Rosenstreich, D.; Ramesh, M. Eosinophilia in Asthma Patients Is Protective Against Severe COVID-19 Illness. J. Allergy Clin. Immunol. Pract. 2021, 9, 1152–1162.e3.
- Ackermann, M.; Anders, H.J.; Bilyy, R.; Bowlin, G.L.; Daniel, C.; De Lorenzo, R.; Egeblad, M.; Henneck, T.; Hidalgo, A.; Hoffmann, M.; et al. Patients with COVID-19: In the dark-NETs of neutrophils. Cell Death Differ. 2021, 28, 3125–3139.
- Basnet, S.; Palmenberg, A.C.; Gern, J.E. Rhinoviruses and Their Receptors. Chest 2019, 155, 1018–1025.
- Bochkov, Y.A.; Gern, J.E. Rhinoviruses and Their Receptors: Implications for Allergic Disease. Curr. Allergy Asthma Rep. 2016, 16, 30.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8.
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422.
- Rath, S.; Perikala, V.; Jena, A.B.; Dandapat, J. Factors regulating dynamics of angiotensin-converting enzyme-2 (ACE2), the gateway of SARS-CoV-2: Epigenetic modifications and therapeutic interventions by epidrugs. Biomed. Pharmacother. 2021, 143, 112095.
- Jackson, D.J.; Busse, W.W.; Bacharier, L.B.; Kattan, M.; O’Connor, G.T.; Wood, R.A.; Visness, C.M.; Durham, S.R.; Larson, D.; Esnault, S.; et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J. Allergy Clin. Immunol. 2020, 146, 203–206.e3.
- Kimura, H.; Francisco, D.; Conway, M.; Martinez, F.D.; Vercelli, D.; Polverino, F.; Billheimer, D.; Kraft, M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J. Allergy Clin. Immunol. 2020, 146, 80–88.e8.
- Camiolo, M.; Gauthier, M.; Kaminski, N.; Ray, A.; Wenzel, S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J. Allergy Clin. Immunol. 2020, 146, 315–324.e7.
- Song, J.; Zeng, M.; Wang, H.; Qin, C.; Hou, H.Y.; Sun, Z.Y.; Xu, S.P.; Wang, G.P.; Guo, C.L.; Deng, Y.K.; et al. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy 2021, 76, 483–496.
- Alashkar Alhamwe, B.; Miethe, S.; Pogge von Strandmann, E.; Potaczek, D.P.; Garn, H. Epigenetic Regulation of Airway Epithelium Immune Functions in Asthma. Front. Immunol. 2020, 11, 1747.
- Bridges, J.P.; Vladar, E.K.; Huang, H.; Mason, R.J. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax 2022, 77, 203–209.
- Lopez, J.; Mommert, M.; Mouton, W.; Pizzorno, A.; Brengel-Pesce, K.; Mezidi, M.; Villard, M.; Lina, B.; Richard, J.C.; Fassier, J.B.; et al. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J. Exp. Med. 2021, 218, e20211211.
- Pizzorno, A.; Padey, B.; Julien, T.; Trouillet-Assant, S.; Traversier, A.; Errazuriz-Cerda, E.; Fouret, J.; Dubois, J.; Gaymard, A.; Lescure, F.X.; et al. Characterization and Treatment of SARS-CoV-2 in Nasal and Bronchial Human Airway Epithelia. Cell Rep. Med. 2020, 1, 100059.
- Zhu, N.; Wang, W.; Liu, Z.; Liang, C.; Wang, W.; Ye, F.; Huang, B.; Zhao, L.; Wang, H.; Zhou, W.; et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020, 11, 3910.
- Potaczek, D.P.; Miethe, S.; Schindler, V.; Alhamdan, F.; Garn, H. Role of airway epithelial cells in the development of different asthma phenotypes. Cell. Signal. 2020, 69, 109523.
- Alashkar Alhamwe, B.; Potaczek, D.P.; Miethe, S.; Alhamdan, F.; Hintz, L.; Magomedov, A.; Garn, H. Extracellular Vesicles and Asthma-More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22, 4984.
- Global Initiative for Asthma. GINA Guidance about COVID-19 and Asthma. Updated 30 March 2021. Available online: https://ginasthma.org/wp-content/uploads/2021/03/21_03_30-GINA-COVID-19-and-asthma.pdf (accessed on 29 October 2021).
- Choi, Y.J.; Park, J.Y.; Lee, H.S.; Suh, J.; Song, J.Y.; Byun, M.K.; Cho, J.H.; Kim, H.J.; Lee, J.H.; Park, J.W.; et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur. Respir. J. 2021, 57, 2002226.
- Pfaar, O.; Klimek, L.; Hamelmann, E.; Kleine-Tebbe, J.; Taube, C.; Wagenmann, M.; Werfel, T.; Brehler, R.; Novak, N.; Mulleneisen, N.; et al. COVID-19 vaccination of patients with allergies and type-2 inflammation with concurrent antibody therapy (biologicals)—A Position Paper of the German Society of Allergology and Clinical Immunology (DGAKI) and the German Society for Applied Allergology (AeDA). Allergol. Select 2021, 5, 140–147.
- Caminati, M.; Guarnieri, G.; Batani, V.; Scarpieri, E.; Finocchiaro, A.; Chieco-Bianchi, F.; Senna, G.; Vianello, A. COVID-19 Vaccination in Patients with Severe Asthma on Biologic Treatment: Safety, Tolerability, and Impact on Disease Control. Vaccines 2021, 9, 853.
- Ramadan, A.A.; Gaffin, J.M.; Israel, E.; Phipatanakul, W. Asthma and Corticosteroid Responses in Childhood and Adult Asthma. Clin. Chest Med. 2019, 40, 163–177.
- FakhriRavari, A.; Jin, S.; Kachouei, F.H.; Le, D.; Lopez, M. Systemic corticosteroids for management of COVID-19: Saving lives or causing harm? Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211063976.
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020, 395, 473–475.
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic review of treatment effects. PLoS Med. 2006, 3, e343.
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease is Suspected. Interim Guidance. 13 March 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/clinical-managementof-novel-cov.pdf (accessed on 1 December 2021).
- World Health Organization. Corticosteroids for COVID-19. Living Guidance, 2 September 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed on 1 December 2021).
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704.
- Yu, L.M.; Bafadhel, M.; Dorward, J.; Hayward, G.; Saville, B.R.; Gbinigie, O.; Van Hecke, O.; Ogburn, E.; Evans, P.H.; Thomas, N.P.B.; et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial. Lancet 2021, 398, 843–855.
- Halpin, D.M.G.; Faner, R.; Sibila, O.; Badia, J.R.; Agusti, A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir. Med. 2020, 8, 436–438.
- McKeever, T.; Harrison, T.W.; Hubbard, R.; Shaw, D. Inhaled corticosteroids and the risk of pneumonia in people with asthma: A case-control study. Chest 2013, 144, 1788–1794.
- Yang, M.; Chen, H.; Zhang, Y.; Du, Y.; Xu, Y.; Jiang, P.; Xu, Z. Long-term use of inhaled corticosteroids and risk of upper respiratory tract infection in chronic obstructive pulmonary disease: A meta-analysis. Inhal. Toxicol. 2017, 29, 219–226.
- Beyerstedt, S.; Casaro, E.B.; Rangel, E.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919.
- Peters, M.C.; Sajuthi, S.; Deford, P.; Christenson, S.; Rios, C.L.; Montgomery, M.T.; Woodruff, P.G.; Mauger, D.T.; Erzurum, S.C.; Johansson, M.W.; et al. COVID-19-related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids. Am. J. Respir. Crit. Care Med. 2020, 202, 83–90.
- Yamaya, M.; Nishimura, H.; Deng, X.; Sugawara, M.; Watanabe, O.; Nomura, K.; Shimotai, Y.; Momma, H.; Ichinose, M.; Kawase, T. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir. Investig. 2020, 58, 155–168.
- Matsuyama, S.; Kawase, M.; Nao, N.; Shirato, K.; Ujike, M.; Kamitani, W.; Shimojima, M.; Fukushi, S. The Inhaled Steroid Ciclesonide Blocks SARS-CoV-2 RNA Replication by Targeting the Viral Replication-Transcription Complex in Cultured Cells. J. Virol. 2020, 95, e01648-20.
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob. Agents Chemother. 2020, 64, e00819-20.
- Oliver, B.G.; Robinson, P.; Peters, M.; Black, J. Viral infections and asthma: An inflammatory interface? Eur. Respir. J. 2014, 44, 1666–1681.
- Ong, K.Y.; Tan, T.L.; Chan, A.K.W.; Tan, K.L.L.; Koh, M.S. Managing asthma in the COVID-19 pandemic and current recommendations from professional bodies: A review. J. Asthma 2021, 58, 1536–1543.
More
